p62 ubiquitin binding-associated domain mediated the receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation: a new insight into the pathogenesis of Paget's disease of bone
- PMID: 16877352
- PMCID: PMC1698794
- DOI: 10.2353/ajpath.2006.050960
p62 ubiquitin binding-associated domain mediated the receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation: a new insight into the pathogenesis of Paget's disease of bone
Abstract
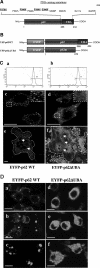
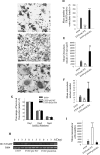
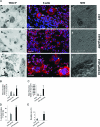
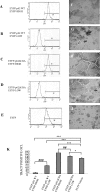
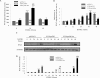
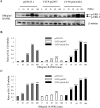
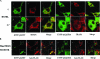
Paget's disease of bone (PDB) is a debilitating bone disorder characterized by giant osteoclasts, enhanced bone destruction, and irregular bone formation. Recently, mutations in SQSTM1 (also known as p62) have been detected in PDB sufferers, with all mutations resulting in either loss of function or truncation/deletion of the ubiquitin binding-associated (UBA) domain. We hypothesized that mutation in the p62 gene resulting in either deletion or premature termination of the UBA domain accounts for the elevated osteoclastic formation and bone resorption associated with PDB. Remarkably, overexpression of the p62 UBA domain deletion mutant (p62DeltaUBA) significantly enhanced osteoclastogenesis in vitro compared to cells expressing either wild-type p62 (p62WT) or a control vector in a RAW264.7 osteoclastogenic system. Overexpression of p62DeltaUBA potentiated the formation of abnormally large multinucleated osteoclasts and resorption of bone, reminiscent of PDB. Consistent with the enhancement of osteoclastogenesis, overexpression of p62DeltaUBA potentiated receptor activator of nuclear factor-kappaB ligand-induced activation of nuclear factor-kappaB, NFAT, and ERK phosphorylation. Furthermore, as determined by confocal microscopy, deletion of the p62 UBA domain impaired the association of p62 with TRAF6 in the proteasomal compartment. These results suggest that the UBA domain encodes essential regulatory elements required for receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation and bone resorption that may be directly associated with the progression of PDB.
Figures
Similar articles
-
A novel mutation (K378X) in the sequestosome 1 gene associated with increased NF-kappaB signaling and Paget's disease of bone with a severe phenotype.J Bone Miner Res. 2006 Jul;21(7):1136-45. doi: 10.1359/jbmr.060405. J Bone Miner Res. 2006. PMID: 16813535
-
Mutant p62P392L stimulation of osteoclast differentiation in Paget's disease of bone.Endocrinology. 2011 Nov;152(11):4180-9. doi: 10.1210/en.2011-1225. Epub 2011 Aug 30. Endocrinology. 2011. PMID: 21878516 Free PMC article.
-
New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget's disease of bone.Endocr Rev. 2013 Aug;34(4):501-24. doi: 10.1210/er.2012-1034. Epub 2013 Apr 23. Endocr Rev. 2013. PMID: 23612225 Review.
-
Sequestosome 1 mutations in Paget's disease of bone in Australia: prevalence, genotype/phenotype correlation, and a novel non-UBA domain mutation (P364S) associated with increased NF-kappaB signaling without loss of ubiquitin binding.J Bone Miner Res. 2009 Jul;24(7):1216-23. doi: 10.1359/jbmr.090214. J Bone Miner Res. 2009. PMID: 19257822
-
Genetics of Paget's disease of bone.Clin Sci (Lond). 2005 Sep;109(3):257-63. doi: 10.1042/CS20050053. Clin Sci (Lond). 2005. PMID: 16104845 Review.
Cited by
-
The adaptor protein p62 is involved in RANKL-induced autophagy and osteoclastogenesis.J Histochem Cytochem. 2014 Dec;62(12):879-88. doi: 10.1369/0022155414551367. Epub 2014 Aug 27. J Histochem Cytochem. 2014. PMID: 25163928 Free PMC article.
-
Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli.J Cell Mol Med. 2019 May;23(5):3077-3087. doi: 10.1111/jcmm.14277. Epub 2019 Mar 20. J Cell Mol Med. 2019. PMID: 30892789 Free PMC article. Review.
-
Erk1 positively regulates osteoclast differentiation and bone resorptive activity.PLoS One. 2011;6(9):e24780. doi: 10.1371/journal.pone.0024780. Epub 2011 Sep 22. PLoS One. 2011. PMID: 21961044 Free PMC article.
-
High Intensity Focused Ultrasound-Driven Nanomotor for Effective Ferroptosis-Immunotherapy of TNBC.Adv Sci (Weinh). 2024 Apr;11(15):e2305546. doi: 10.1002/advs.202305546. Epub 2024 Feb 11. Adv Sci (Weinh). 2024. PMID: 38342612 Free PMC article.
-
Nitidine chloride prevents OVX-induced bone loss via suppressing NFATc1-mediated osteoclast differentiation.Sci Rep. 2016 Nov 8;6:36662. doi: 10.1038/srep36662. Sci Rep. 2016. PMID: 27821837 Free PMC article.
References
-
- Roodman GD. Mechanisms of abnormal bone turnover in Paget’s disease. Bone. 1999;24:39S–40S. - PubMed
-
- Reddy SV. Etiology of Paget’s disease and osteoclast abnormalities. J Cell Biochem. 2004;93:688–696. - PubMed
-
- Layfield R, Hocking LJ. SQSTM1 and Paget’s disease of bone. Calcif Tissue Int. 2004;75:347–357. - PubMed
-
- Morgan SL, Ward L, Ward BK, Walsh J, Xu J: A novel mutation (K378X) in the sequestosome1 gene associated with a sever phenotype in Paget’s disease of bone. International Symposium on Paget’s Disease, 2005 July 8–9, St. Catherine’s College, Oxford, UK - PubMed
-
- Hocking LJ, Lucas GJ, Daroszewska A, Cundy T, Nicholson GC, Donath J, Walsh JP, Finlayson C, Cavey JR, Ciani B, Sheppard PW, Searle MS, Layfield R, Ralston SH. Novel UBA domain mutations of SQSTM1 in Paget’s disease of bone: genotype phenotype correlation, functional analysis, and structural consequences. J Bone Miner Res. 2004;19:1122–1127. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

