NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1
- PMID: 16877356
- PMCID: PMC1698792
- DOI: 10.2353/ajpath.2006.051067
NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1
Abstract
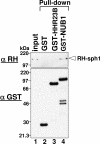
NUB1 is a potent down-regulator of the ubiquitin-like protein NEDD8, because it targets NEDD8 to the proteasome for proteolytic degradation. From results in this study, we found that NUB1 physically interacts with synphilin-1 through its NEDD8-binding site, implying that NUB1 also targets synphilin-1 to the proteasome for degradation. Synphilin-1 is a major component of inclusion bodies found in the brains of patients with neurodegenerative alpha-synucleinopathies, including Parkinson's disease. In this study, we immunostained sections of brains from patients with Parkinson's disease and other alpha-synucleinopathies and demonstrated that NUB1, as well as synphilin-1, accumulates in the inclusion bodies. To define the role of NUB1 in the formation of these inclusion bodies, we performed a co-transfection assay using cultured HEK293 cells. This assay showed that NUB1 suppresses the formation of synphilin-1-positive inclusions. Further, biochemical assays revealed that NUB1 overexpression leads to the proteasomal degradation of synphilin-1. These results and our previous observations suggest that NUB1 indeed targets synphilin-1 to the proteasome for its efficient degradation, which, because of the resultant reduction in synphilin-1, suppresses the formation of synphilin-1-positive inclusions.
Figures






References
-
- Kamitani T, Kito K, Nguyen HP, Yeh ETH. Characterization of NEDD8, a developmentally down-regulated ubiquitin-like molecule. J Biol Chem. 1997;272:28557–28562. - PubMed
-
- Wada H, Yeh ETH, Kamitani T. Identification of NEDD8-conjugation site in human cullin-2. Biochem Biophys Res Commun. 1999;257:100–105. - PubMed
-
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. - PubMed
-
- Yeh ETH, Gong L, Kamitani T. Ubiquitin-like proteins: new wines in new bottles. Gene. 2000;248:1–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

