Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation
- PMID: 16877368
- PMCID: PMC1764216
- DOI: 10.2353/ajpath.2006.051200
Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation
Abstract
Lymphatic vessel plasticity and stability are of considerable importance when attempting to treat diseases associated with the lymphatic vasculature. Development of lymphatic vessels during embryogenesis is dependent on vascular endothelial growth factor (VEGF)-C but not VEGF-D. Using a recombinant adenovirus encoding a soluble form of their receptor VEGFR-3 (AdVEGFR-3-Ig), we studied lymphatic vessel dependency on VEGF-C and VEGF-D induced VEGFR-3 signaling in postnatal and adult mice. Transduction with AdVEGFR-3-Ig led to regression of lymphatic capillaries and medium-sized lymphatic vessels in mice under 2 weeks of age without affecting collecting lymphatic vessels or the blood vasculature. No effect was observed after this period. The lymphatic capillaries of neonatal mice also regressed partially in response to recombinant VEGFR-3-Ig or blocking antibodies against VEGFR-3, but not to adenovirus-encoded VEGFR-2-Ig. Despite sustained inhibitory VEGFR-3-Ig levels, lymphatic vessel regrowth was observed at 4 weeks of age. Interestingly, whereas transgenic expression of VEGF-C in the skin induced lymphatic hyperplasia even during embryogenesis, similar expression of VEGF-D resulted in lymphangiogenesis predominantly after birth. These results indicate considerable plasticity of lymphatic vessels during the early postnatal period but not thereafter, suggesting that anti-lymphangiogenic therapy can be safely applied in adults.
Figures
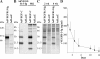
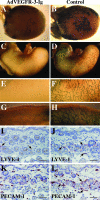

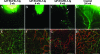


Comment in
-
When it comes to blocking lymphatics, it is all a question of time.Am J Pathol. 2006 Aug;169(2):347-50. doi: 10.2353/ajpath.2006.060427. Am J Pathol. 2006. PMID: 16877337 Free PMC article. No abstract available.
Similar articles
-
Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues.Am J Pathol. 2008 Dec;173(6):1891-901. doi: 10.2353/ajpath.2008.080378. Epub 2008 Nov 6. Am J Pathol. 2008. PMID: 18988807 Free PMC article.
-
Lymphangiogenic growth factors, receptors and therapies.Thromb Haemost. 2003 Aug;90(2):167-84. doi: 10.1160/TH03-04-0200. Thromb Haemost. 2003. PMID: 12888864 Review.
-
VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study.Angiogenesis. 2015 Jul;18(3):313-26. doi: 10.1007/s10456-015-9469-2. Epub 2015 May 28. Angiogenesis. 2015. PMID: 26018927
-
Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice.EMBO J. 2001 Mar 15;20(6):1223-31. doi: 10.1093/emboj/20.6.1223. EMBO J. 2001. PMID: 11250889 Free PMC article.
-
VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels.Dev Dyn. 2015 Mar;244(3):323-31. doi: 10.1002/dvdy.24227. Epub 2014 Dec 4. Dev Dyn. 2015. PMID: 25399804 Review.
Cited by
-
Regulation of endothelial cell differentiation and specification.Circ Res. 2013 Apr 26;112(9):1272-87. doi: 10.1161/CIRCRESAHA.113.300506. Circ Res. 2013. PMID: 23620236 Free PMC article. Review.
-
Podoplanin-Fc reduces lymphatic vessel formation in vitro and in vivo and causes disseminated intravascular coagulation when transgenically expressed in the skin.Blood. 2010 Nov 18;116(20):4376-84. doi: 10.1182/blood-2010-04-278564. Epub 2010 Aug 17. Blood. 2010. PMID: 20716773 Free PMC article.
-
Developmental and pathological lymphangiogenesis: from models to human disease.Histochem Cell Biol. 2008 Dec;130(6):1063-78. doi: 10.1007/s00418-008-0525-5. Epub 2008 Oct 23. Histochem Cell Biol. 2008. PMID: 18946678 Review.
-
Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth.Nat Med. 2009 Sep;15(9):1023-30. doi: 10.1038/nm.2018. Epub 2009 Aug 9. Nat Med. 2009. PMID: 19668192 Free PMC article.
-
Targeted Therapy for Complex Lymphatic Anomalies in Patients with Noonan Syndrome and Related Disorders.Int J Mol Sci. 2025 Jun 26;26(13):6126. doi: 10.3390/ijms26136126. Int J Mol Sci. 2025. PMID: 40649904 Free PMC article.
References
-
- Oliver G, Alitalo K. The lymphatic vasculature: recent progress and paradigms. Annu Rev Cell Dev Biol. 2005;21:457–483. - PubMed
-
- Jeltsch M, Tammela T, Alitalo K, Wilting J. Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res. 2003;314:69–84. - PubMed
-
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. - PubMed
-
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

