Glutathiolation enhances the degradation of gammaC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway
- PMID: 16877417
- PMCID: PMC2117893
- DOI: 10.1167/iovs.05-1664
Glutathiolation enhances the degradation of gammaC-crystallin in lens and reticulocyte lysates, partially via the ubiquitin-proteasome pathway
Abstract
Purpose: S-glutathiolated proteins are formed in the lens during aging and cataractogenesis. The objective of this work was to explore the role of the ubiquitin-proteasome pathway in eliminating S-glutathiolated gammaC-crystallin.
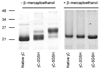
Methods: Recombinant human gammaC-crystallin was mixed with various concentrations of glutathione (GSH) and diamide at 25 degrees C for 1 hour. The extent of glutathiolation of the gammaC-crystallin was determined by mass spectrometry. Native and S-glutathiolated gammaC-crystallins were labeled with (125)I, and proteolytic degradation was determined using both lens fiber lysate and reticulocyte lysate as sources of ubiquitinating and proteolytic enzymes. Far UV circular dichroism, tryptophan fluorescence intensity, and binding to the hydrophobic fluorescence probe 4,4'-dianilino-1,1'-binaphthalene-5,5'-disulfonic acid (Bis-ANS), were used to characterize the native and glutathiolated gammaC-crystallins.
Results: On average, two and five of the eight cysteines in gammaC-crystallin were glutathiolated when molar ratios of gammaC-crystallin-GSH-diamide were 1:2:5 and 1:10:25, respectively. Native gammaC-crystallin was resistant to degradation in both lens fiber lysate and reticulocyte lysate. However, glutathiolated gammaC-crystallin showed a significant increase in proteolytic degradation in both lens fiber and reticulocyte lysates. Proteolysis was stimulated by addition of adenosine triphosphate (ATP) and Ubc4 and was substantially inhibited by the proteasome inhibitor MG132 and a dominant negative form of ubiquitin, indicating that at least part of the proteolysis was mediated by the ubiquitin-proteasome pathway. Spectroscopic analyses of glutathiolated gammaC-crystallin revealed conformational changes and partial unfolding, which may provide a signal for the ubiquitin-dependent degradation.
Conclusions: The present data demonstrate that oxidative modification by glutathiolation can render lens proteins more susceptible to degradation by the ubiquitin-proteasome pathway. Together with previous results, these data support the concept that the ubiquitin-proteasome pathway serves as a general protein quality-control mechanism.
Figures





Similar articles
-
Degradation of C-terminal truncated alpha A-crystallins by the ubiquitin-proteasome pathway.Invest Ophthalmol Vis Sci. 2007 Sep;48(9):4200-8. doi: 10.1167/iovs.07-0196. Invest Ophthalmol Vis Sci. 2007. PMID: 17724207 Free PMC article.
-
Glutathiolation Triggers Proteins for Degradation by the Ubiquitin- Proteasome Pathway.Curr Mol Med. 2017 Dec 7;17(4):258-269. doi: 10.2174/1566524017666171101165021. Curr Mol Med. 2017. PMID: 29110605
-
Spectroscopic analysis of lens recombinant betaB2- and gammaC-crystallin.Mol Vis. 2001 Jul 26;7:178-83. Mol Vis. 2001. PMID: 11483894
-
The Functional Significance of High Cysteine Content in Eye Lens γ-Crystallins.Biomolecules. 2024 May 17;14(5):594. doi: 10.3390/biom14050594. Biomolecules. 2024. PMID: 38786000 Free PMC article. Review.
-
Measurement and identification of S-glutathiolated proteins.Methods Enzymol. 2010;473:179-97. doi: 10.1016/S0076-6879(10)73009-3. Methods Enzymol. 2010. PMID: 20513478 Free PMC article. Review.
Cited by
-
Macrophage-restricted overexpression of glutaredoxin 1 protects against atherosclerosis by preventing nutrient stress-induced macrophage dysfunction and reprogramming.Atherosclerosis. 2023 Dec;387:117383. doi: 10.1016/j.atherosclerosis.2023.117383. Epub 2023 Nov 22. Atherosclerosis. 2023. PMID: 38061313 Free PMC article.
-
Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: implications in the pathogenesis of age-related macular degeneration.Mol Aspects Med. 2012 Aug;33(4):446-66. doi: 10.1016/j.mam.2012.04.001. Epub 2012 Apr 10. Mol Aspects Med. 2012. PMID: 22521794 Free PMC article. Review.
-
Expression of K6W-ubiquitin in lens epithelial cells leads to upregulation of a broad spectrum of molecular chaperones.Mol Vis. 2008 Mar 4;14:403-12. Mol Vis. 2008. PMID: 18334961 Free PMC article.
-
Oligomerization with wt αA- and αB-crystallins reduces proteasome-mediated degradation of C-terminally truncated αA-crystallin.Invest Ophthalmol Vis Sci. 2012 May 4;53(6):2541-50. doi: 10.1167/iovs.11-9147. Invest Ophthalmol Vis Sci. 2012. PMID: 22427585 Free PMC article.
-
Glycative stress as a cause of macular degeneration.Prog Retin Eye Res. 2024 Jul;101:101260. doi: 10.1016/j.preteyeres.2024.101260. Epub 2024 Mar 21. Prog Retin Eye Res. 2024. PMID: 38521386 Free PMC article. Review.
References
-
- Bloemendal H, de Jong W, Jaenicke R, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. - PubMed
-
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. - PubMed
-
- Lampi KJ, Ma Z, Hanson SR, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. - PubMed
-
- Zarina S, Zhao HR, Abraham EC. Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem. 2000;210:29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

