Single hepatitis-B virus core capsid binding to individual nuclear pore complexes in Hela cells
- PMID: 16877503
- PMCID: PMC1578495
- DOI: 10.1529/biophysj.106.087650
Single hepatitis-B virus core capsid binding to individual nuclear pore complexes in Hela cells
Abstract
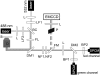
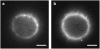
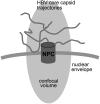
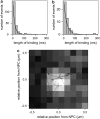
We investigate the interaction of hepatitis B virus capsids lacking a nuclear localization signal with nuclear pore complexes (NPCs) in permeabilized HeLa cells. Confocal and wide-field optical images of the nuclear envelope show well-spaced individual NPCs. Specific interactions of capsids with single NPCs are characterized by extended residence times of capsids in the focal volume which are characterized by fluorescence correlation spectroscopy. In addition, single-capsid-tracking experiments using fast wide-field fluorescence microscopy at 50 frames/s allow us to directly observe specific binding via a dual-color colocalization of capsids and NPCs. We find that binding occurs with high probability on the nuclear-pore ring moiety, at 44 +/- 9 nm radial distance from the central axis.
Figures

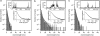

Similar articles
-
Signals for bidirectional nucleocytoplasmic transport in the duck hepatitis B virus capsid protein.J Virol. 2001 Feb;75(4):1968-77. doi: 10.1128/JVI.75.4.1968-1977.2001. J Virol. 2001. PMID: 11160696 Free PMC article.
-
Nuclear transport of single molecules: dwell times at the nuclear pore complex.J Cell Biol. 2005 Jan 17;168(2):233-43. doi: 10.1083/jcb.200411005. J Cell Biol. 2005. PMID: 15657394 Free PMC article.
-
Targeting of viral capsids to nuclear pores in a cell-free reconstitution system.Traffic. 2014 Nov;15(11):1266-81. doi: 10.1111/tra.12209. Epub 2014 Sep 12. Traffic. 2014. PMID: 25131140 Free PMC article.
-
Nuclear Import of Hepatitis B Virus Capsids and Genome.Viruses. 2017 Jan 21;9(1):21. doi: 10.3390/v9010021. Viruses. 2017. PMID: 28117723 Free PMC article. Review.
-
Nuclear Import of Adeno-Associated Viruses Imaged by High-Speed Single-Molecule Microscopy.Viruses. 2021 Jan 22;13(2):167. doi: 10.3390/v13020167. Viruses. 2021. PMID: 33499411 Free PMC article. Review.
Cited by
-
MPTHub: An Open-Source Software for Characterizing the Transport of Particles in Biorelevant Media.Nanomaterials (Basel). 2022 Jun 1;12(11):1899. doi: 10.3390/nano12111899. Nanomaterials (Basel). 2022. PMID: 35683754 Free PMC article.
-
Misdelivery at the Nuclear Pore Complex-Stopping a Virus Dead in Its Tracks.Cells. 2015 Jul 28;4(3):277-96. doi: 10.3390/cells4030277. Cells. 2015. PMID: 26226003 Free PMC article. Review.
-
Many mechanisms, one entrance: membrane protein translocation into the nucleus.Cell Mol Life Sci. 2012 Jul;69(13):2205-16. doi: 10.1007/s00018-012-0929-1. Epub 2012 Feb 12. Cell Mol Life Sci. 2012. PMID: 22327555 Free PMC article. Review.
-
Intracellular Trafficking of HBV Particles.Cells. 2020 Sep 2;9(9):2023. doi: 10.3390/cells9092023. Cells. 2020. PMID: 32887393 Free PMC article. Review.
References
-
- Crowther, R. A., N. A. Kiselev, B. Bottcher, J. A. Berriman, G. P. Borisova, V. Ose, and P. Pumpens. 1994. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell. 77:943–950. - PubMed
-
- Stoffler, D., B. Feja, B. Fahrenkrog, J. Walz, D. Typke, and U. Aebi. 2003. Cryo-electron tomography provides novel insights into nuclear pore architecture: implications for nucleocytoplasmic transport. J. Mol. Biol. 328:119–130. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

