Interaction of verapamil with lipid membranes and P-glycoprotein: connecting thermodynamics and membrane structure with functional activity
- PMID: 16877510
- PMCID: PMC1578493
- DOI: 10.1529/biophysj.106.089581
Interaction of verapamil with lipid membranes and P-glycoprotein: connecting thermodynamics and membrane structure with functional activity
Abstract
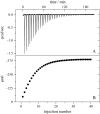
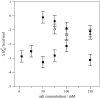
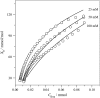
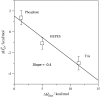
Verapamil and amlodipine are calcium ion influx inhibitors of wide clinical use. They are partially charged at neutral pH and exhibit amphiphilic properties. The noncharged species can easily cross the lipid membrane. We have measured with solid-state NMR the structural changes induced by verapamil upon incorporation into phospholipid bilayers and have compared them with earlier data on amlodipine and nimodipine. Verapamil and amlodipine produce a rotation of the phosphocholine headgroup away from the membrane surface and a disordering of the fatty acid chains. We have determined the thermodynamics of verapamil partitioning into neutral and negatively charged membranes with isothermal titration calorimetry. Verapamil undergoes a pK-shift of DeltapK(a) = 1.2 units in neutral lipid membranes and the percentage of the noncharged species increases from 5% to 45%. Verapamil partitioning is increased for negatively charged membranes and the binding isotherms are strongly affected by the salt concentration. The electrostatic screening can be explained with the Gouy-Chapman theory. Using a functional phosphate assay we have measured the affinity of verapamil, amlodipine, and nimodipine for P-glycoprotein, and have calculated the free energy of drug binding from the aqueous phase to the active center of P-glycoprotein in the lipid phase. By combining the latter results with the lipid partitioning data it was possible, for the first time, to determine the true affinity of the three drugs for the P-glycoprotein active center if the reaction takes place exclusively in the lipid matrix.
Figures





References
-
- Romsicki, Y., and F. J. Sharom. 1999. The membrane lipid environment modulates drug interactions with the P-glycoprotein multidrug transporter. Biochemistry. 38:6887–6896. - PubMed
-
- Higgins, C. F., and M. M. Gottesman. 1992. Is the multidrug transporter a flippase? Trends Biochem. Sci. 17:18–21. - PubMed
-
- Lu, P., R. Liu, and F. J. Sharom. 2001. Drug transport by reconstituted P-glycoprotein in proteoliposomes. Effect of substrates and modulators, and dependence on bilayer phase state. Eur. J. Biochem. 268:1687–1697. - PubMed
-
- Seelig, A., and E. Gatlik-Landwojtowicz. 2005. Inhibitors of multidrug efflux transporters: their membrane and protein interactions. Mini Rev. Med. Chem. 5:135–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

