Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries
- PMID: 16878174
- PMCID: PMC1518791
- DOI: 10.1172/JCI26605
Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries
Erratum in
- J Clin Invest. 2006 Sep;116(9):2562
Abstract
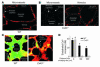
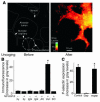
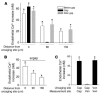
Acute lung injury (ALI), which is associated with a mortality of 30-40%, is attributable to inflammation that develops rapidly across the lung's vast vascular surface, involving an entire lung or even both lungs. No specific mechanism explains this extensive inflammatory spread, probably because of the lack of approaches for detecting signal conduction in lung capillaries. Here, we addressed this question by applying the photolytic uncaging approach to induce focal increases in Ca2+ levels in targeted endothelial cells of alveolar capillaries. Uncaging caused Ca2+ levels to increase not only in the targeted cell, but also in vascular locations up to 150 microm from the target site, indicating that Ca2+ was conducted from the capillary to adjacent vessels. No such conduction was evident in mouse lungs lacking endothelial connexin 43 (Cx43), or in rat lungs in which we pretreated vessels with peptide inhibitors of Cx43. These findings provide the first direct evidence to our knowledge that interendothelial Ca2+ conduction occurs in the lung capillary bed and that Cx43-containing gap junctions mediate the conduction. A proinflammatory effect was evident in that induction of increases in Ca2+ levels in the capillary activated expression of the leukocyte adherence receptor P-selectin in venules. Further, peptide inhibitors of Cx43 completely blocked thrombin-induced microvascular permeability increases. Together, our findings reveal a novel role for Cx43-mediated gap junctions, namely as conduits for the spread of proinflammatory signals in the lung capillary bed. Gap junctional mechanisms require further consideration in the understanding of ALI.
Figures
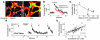
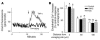
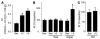
Similar articles
-
Endothelial connexin43 mediates acid-induced increases in pulmonary microvascular permeability.Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L33-42. doi: 10.1152/ajplung.00219.2011. Epub 2012 May 4. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22561459 Free PMC article.
-
Pressure is proinflammatory in lung venular capillaries.J Clin Invest. 1999 Aug;104(4):495-502. doi: 10.1172/JCI6872. J Clin Invest. 1999. PMID: 10449441 Free PMC article.
-
Direct cell/cell communication in the lymphoid germinal center: connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes.Eur J Immunol. 1997 Jun;27(6):1489-97. doi: 10.1002/eji.1830270627. Eur J Immunol. 1997. PMID: 9209502
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
-
Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels.Neuropharmacology. 2013 Dec;75:491-505. doi: 10.1016/j.neuropharm.2013.04.050. Epub 2013 May 7. Neuropharmacology. 2013. PMID: 23664811 Review.
Cited by
-
The Role of Connexin Hemichannels in Inflammatory Diseases.Biology (Basel). 2022 Feb 2;11(2):237. doi: 10.3390/biology11020237. Biology (Basel). 2022. PMID: 35205103 Free PMC article. Review.
-
Ca²⁺-dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients.BMC Surg. 2013;13 Suppl 2(Suppl 2):S40. doi: 10.1186/1471-2482-13-S2-S40. Epub 2013 Oct 8. BMC Surg. 2013. PMID: 24266895 Free PMC article.
-
Novel regulators of endothelial barrier function.Am J Physiol Lung Cell Mol Physiol. 2014 Dec 15;307(12):L924-35. doi: 10.1152/ajplung.00318.2014. Epub 2014 Nov 7. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25381026 Free PMC article. Review.
-
Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN.J Am Soc Nephrol. 2017 Oct;28(10):2915-2930. doi: 10.1681/ASN.2016111211. Epub 2017 Jun 30. J Am Soc Nephrol. 2017. PMID: 28667079 Free PMC article.
-
Contribution of Connexin Hemichannels to the Pathogenesis of Acute Lung Injury.Mediators Inflamm. 2020 Nov 17;2020:8094347. doi: 10.1155/2020/8094347. eCollection 2020. Mediators Inflamm. 2020. PMID: 33293898 Free PMC article.
References
-
- MacCallum N.S., Evans T.W. Epidemiology of acute lung injury. Curr. Opin. Crit. Care. 2005;11:43–49. - PubMed
-
- Rubenfeld G.D., et al. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. - PubMed
-
- Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. - PubMed
-
- Parthasarathi K., Ichimura H., Quadri S., Issekutz A., Bhattacharya J. Mitochondrial reactive oxygen species regulate spatial profile of proinflammatory responses in lung venular capillaries. J. Immunol. 2002;169:7078–7086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

