Glucocorticoids suppress bone formation via the osteoclast
- PMID: 16878176
- PMCID: PMC1518793
- DOI: 10.1172/JCI28084
Glucocorticoids suppress bone formation via the osteoclast
Abstract
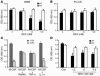
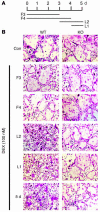
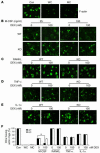
The pathogenesis of glucocorticoid-induced (GC-induced) bone loss is unclear. For example, osteoblast apoptosis is enhanced by GCs in vivo, but they stimulate bone formation in vitro. This conundrum suggests that an intermediary cell transmits a component of the bone-suppressive effects of GCs to osteoblasts in the intact animal. Bone remodeling is characterized by tethering of the activities of osteoclasts and osteoblasts. Hence, the osteoclast is a potential modulator of the effect of GCs on osteoblasts. To define the direct impact of GCs on bone-resorptive cells, we compared the effects of dexamethasone (DEX) on WT osteoclasts with those derived from mice with disruption of the GC receptor in osteoclast lineage cells (GRoc-/- mice). While the steroid prolonged longevity of osteoclasts, their bone-degrading capacity was suppressed. The inhibitory effect of DEX on bone resorption reflects failure of osteoclasts to organize their cytoskeleton in response to M-CSF. DEX specifically arrested M-CSF activation of RhoA, Rac, and Vav3, each of which regulate the osteoclast cytoskeleton. In all circumstances GRoc-/- mice were spared the impact of DEX on osteoclasts and their precursors. Consistent with osteoclasts modulating the osteoblast-suppressive effect of DEX, GRoc-/- mice are protected from the steroid's inhibition of bone formation.
Figures

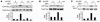



References
-
- Weinstein R.S. Glucocorticoid-induced osteoporosis. Rev. Endocr. Metab. Disord. 2001;2:65–73. - PubMed
-
- Pereira R.M., Delany A.M., Canalis E. Cortisol inhibits the differentiation and apoptosis of osteoblasts in culture. Bone. 2001;28:484–490. - PubMed
-
- Pereira R.C., Delany A.M., Canalis E. Effects of cortisol and bone morphogenetic protein-2 on stromal cell differentiation: correlation with CCAAT-enhancer binding protein expression. Bone. 2002;30:685–691. - PubMed
-
- Aubin J.E. Osteoprogenitor cell frequency in rat bone marrow stromal populations: role for heterotypic cell-cell interactions in osteoblast differentiation. J. Cell. Biochem. 1999;72:396–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK056341/DK/NIDDK NIH HHS/United States
- AR032788/AR/NIAMS NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- AR046852/AR/NIAMS NIH HHS/United States
- AR046523/AR/NIAMS NIH HHS/United States
- AR048853/AR/NIAMS NIH HHS/United States
- R01 AR048853/AR/NIAMS NIH HHS/United States
- R01 AI050655/AI/NIAID NIH HHS/United States
- R21 AI050655/AI/NIAID NIH HHS/United States
- AR048812/AR/NIAMS NIH HHS/United States
- AI50655/AI/NIAID NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 AR046852/AR/NIAMS NIH HHS/United States
- R01 AR032788/AR/NIAMS NIH HHS/United States
- R01 AR048812/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

