Myosin heavy chain isoform transitions in canine skeletal muscles during postnatal growth
- PMID: 16879596
- PMCID: PMC2100321
- DOI: 10.1111/j.1469-7580.2006.00599.x
Myosin heavy chain isoform transitions in canine skeletal muscles during postnatal growth
Abstract
To gain a better understanding of the normal characteristics of developing canine muscles, myosin heavy chain (MHC) isoform expression was analysed in the axial and limb skeletal muscles of 18 young dogs whose ages ranged from the late prenatal stage to 6 months. We compared the results of immunohistochemistry using ten monoclonal antibodies, specific to different MHC isoforms, and enzyme-histochemical reactions, which demonstrate the activity of myofibrillar ATPase, succinate dehydrogenase (SDH) and alpha-glycerophosphate dehydrogenase (alpha-GPDH). In the skeletal muscles of fetuses and neonatal dogs the developmental isoforms MHC-emb and MHC-neo were prevalent. In all muscles the primary fibres, located centrally in each muscle fascicle, strongly expressed the slow isoform MHC-I. The adult fast isoform MHC-IIa was first noted in some of the secondary fibres on fetal day 55. During the first 10 days after birth, the expression of MHC-emb declined, as did that of MHC-neo during the second and third weeks. Correspondingly, the expression of MHC-IIa, and later, of MHC-I increased in the secondary fibres. Between the sixth week and second month the expression of MHC-IIx became prominent. The slow rhomboideus muscle exhibited an early expression of the slow isoform in the secondary fibres. Our results indicate that the timing of muscle maturation depends on its activity immediately following birth. The fastest developing muscle was the diaphragm, followed by the fast muscles. A pronounced changeover from developmental to adult isoforms was noted at 4-6 weeks of age, which coincides with the increased physical activity of puppies.
Figures

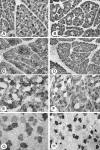
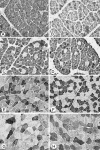




References
-
- Acevedo LM, Rivero JL. New insights into skeletal muscle fibre types in the dog with particular focus towards hybrid myosin phenotypes. Cell Tissue Res. 2005;323:283–303. - PubMed
-
- d’Albis A, Couteaux R, Janmot C, Mira JC. Myosin isoform transitions in regeneration of fast and slow muscles during postnatal development of the rat. Dev Biol. 1989a;135:320–325. - PubMed
-
- d’Albis A, Couteaux R, Janmot C, Roulet A. Specific programs of myosin expression in the postnatal development of rat muscles. Eur J Biochem. 1989b;183:583–590. - PubMed
-
- d’Albis A, Janmot C, Couteaux R. Species- and muscle type-dependence of perinatal isomyosin transitions. Int J Dev Biol. 1991;35:53–56. - PubMed
-
- Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

