Total sleep deprivation inhibits the neuronal nitric oxide synthase and cytochrome oxidase reactivities in the nodose ganglion of adult rats
- PMID: 16879602
- PMCID: PMC2100318
- DOI: 10.1111/j.1469-7580.2006.00594.x
Total sleep deprivation inhibits the neuronal nitric oxide synthase and cytochrome oxidase reactivities in the nodose ganglion of adult rats
Abstract
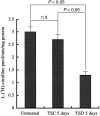
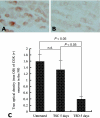
Sleep disorders are a form of stress associated with increased sympathetic activity, and they are a risk factor for the occurrence of cardiovascular disease. Given that nitric oxide (NO) may play an inhibitory role in the regulation of sympathetic tone, this study set out to determine the NO synthase (NOS) reactivity in the primary cardiovascular afferent neurons (i.e. nodose neurons) following total sleep deprivation (TSD). TSD was performed by the disc-on-water method. Following 5 days of TSD, all experimental animals were investigated for quantitative nicotinamine adenine dinucleotide phosphate-diaphorase (NADPH-d, a co-factor of NOS) histochemistry, neuronal NOS immunohistochemistry and neuronal NOS activity assay. In order to evaluate the endogenous metabolic activity of nodose neurons, cytochrome oxidase (COX) reactivity was further tested. All the above-mentioned reactivities were objectively assessed by computerized image analysis. The clinical significance of the reported changes was demonstrated by alterations of mean arterial blood pressure (MAP). The results indicated that in normal untreated rats, numerous NADPH-d/NOS- and COX-reactive neurons were found in the nodose ganglion (NG). Following TSD, however, both the labelling and staining intensity of NADPH-d/NOS as well as COX reactivity were drastically reduced in the NG compared with normal untreated ganglions. MAP was significantly higher in TSD rats (136+/-4 mmHg) than in normal untreated rats (123+/-2 mmHg). NO may serve as an important sympathoinhibition messenger released by the NG neurons, and decrease of NOS immunoexpression following TSD may account for the decrease in NOS content. In association with the reduction of NOS activity, a defect in NOS expression in the primary cardiovascular afferent neurons would enhance clinical hypertension, which might serve as a potential risk factor in the development of TSD-relevant cardiovascular disturbances.
Figures




Similar articles
-
Neuronal NADPH-d/NOS expression in the nodose ganglion of severe hypoxic rats with or without mild hypoxic preconditioning.J Chem Neuroanat. 2005 Mar;29(2):149-56. doi: 10.1016/j.jchemneu.2004.11.001. J Chem Neuroanat. 2005. PMID: 15652701
-
Melatonin restores the cytochrome oxidase reactivity in the nodose ganglia of acute hypoxic rats.J Pineal Res. 2005 Sep;39(2):206-14. doi: 10.1111/j.1600-079X.2005.00238.x. J Pineal Res. 2005. PMID: 16098100
-
Melatonin attenuates the neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats.J Pineal Res. 2002 Mar;32(2):65-73. doi: 10.1034/j.1600-079x.2002.1816.x. J Pineal Res. 2002. PMID: 12071470
-
Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats.J Pineal Res. 2009 Oct;47(3):211-20. doi: 10.1111/j.1600-079X.2009.00704.x. Epub 2009 Jul 21. J Pineal Res. 2009. PMID: 19627456
-
Upregulation of NMDA receptor and neuronal NADPH-d/NOS expression in the nodose ganglion of acute hypoxic rats.J Chem Neuroanat. 2003 Feb;25(2):137-47. doi: 10.1016/s0891-0618(02)00101-1. J Chem Neuroanat. 2003. PMID: 12663061
Cited by
-
Total sleep deprivation alters endothelial function in rats: a nonsympathetic mechanism.Sleep. 2014 Mar 1;37(3):465-73. doi: 10.5665/sleep.3476. Sleep. 2014. PMID: 24587568 Free PMC article.
-
Blood pressure increases during a simulated night shift in persons at risk for hypertension.Int J Behav Med. 2010 Dec;17(4):314-20. doi: 10.1007/s12529-010-9117-6. Int J Behav Med. 2010. PMID: 20878512
-
Light, Water, and Melatonin: The Synergistic Regulation of Phase Separation in Dementia.Int J Mol Sci. 2023 Mar 19;24(6):5835. doi: 10.3390/ijms24065835. Int J Mol Sci. 2023. PMID: 36982909 Free PMC article. Review.
-
Sleep deprivation and oxidative stress in animal models: a systematic review.Oxid Med Cell Longev. 2015;2015:234952. doi: 10.1155/2015/234952. Epub 2015 Apr 6. Oxid Med Cell Longev. 2015. PMID: 25945148 Free PMC article.
-
Sleep deprivation reduces the baroreflex sensitivity through elevated angiotensin (Ang) II subtype 1 receptor expression in the nucleus tractus solitarii.Front Neurosci. 2024 Apr 29;18:1401530. doi: 10.3389/fnins.2024.1401530. eCollection 2024. Front Neurosci. 2024. PMID: 38741786 Free PMC article.
References
-
- Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res. 1995;693:51–63. - PubMed
-
- Augustyniak RA, Thomas GD, Victor RG, Zhang W. Nitric oxide pathway as new drug targets for refractory hypertension. Curr Pharm Des. 2005;11:3307–3315. - PubMed
-
- Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. - PubMed
-
- Blessing WW. Lower brain stem regulation of visceral, cardiovascular, and respiratory function. In: Paxinos G, Mai JK, editors. The Human Nervous System. San Diego: Academic Press; 2004. pp. 295–296.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

