The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis
- PMID: 16880255
- PMCID: PMC2118377
- DOI: 10.1084/jem.20052085
The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgammaIIA receptor-induced phagocytosis
Abstract
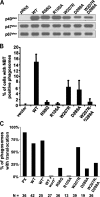
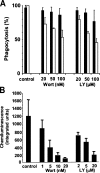
Superoxide produced by the phagocyte reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is essential for host defense. Enzyme activation requires translocation of p67(phox), p47(phox), and Rac-GTP to flavocytochrome b558 in phagocyte membranes. To examine the regulation of phagocytosis-induced superoxide production, flavocytochrome b558, p47(phox), p67(phox), and the FcgammaIIA receptor were expressed from stable transgenes in COS7 cells. The resulting COS(phox)FcgammaR cells produce high levels of superoxide when stimulated with phorbol ester and efficiently ingest immunoglobulin (Ig)G-coated erythrocytes, but phagocytosis did not activate the NADPH oxidase. COS7 cells lack p40(phox), whose role in the NADPH oxidase is poorly understood. p40(phox) contains SH3 and phagocyte oxidase and Bem1p (PB1) domains that can mediate binding to p47(phox) and p67(phox), respectively, along with a PX domain that binds to phosphatidylinositol-3-phosphate (PI(3)P), which is generated in phagosomal membranes. Expression of p40(phox) was sufficient to activate superoxide production in COS(phox)FcgammaR phagosomes. FcgammaIIA-stimulated NADPH oxidase activity was abrogated by point mutations in p40(phox) that disrupt PI(3)P binding, or by simultaneous mutations in the SH3 and PB1 domains. Consistent with an essential role for PI(3)P in regulating the oxidase complex, phagosome NADPH oxidase activation in primary macrophages ingesting IgG-coated beads was inhibited by phosphatidylinositol 3 kinase inhibitors to a much greater extent than phagocytosis itself. Hence, this study identifies a role for p40(phox) and PI(3)P in coupling FcgammaR-mediated phagocytosis to activation of the NADPH oxidase.
Figures
Similar articles
-
Fc gamma R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome.Blood. 2008 Nov 1;112(9):3867-77. doi: 10.1182/blood-2007-11-126029. Epub 2008 Aug 18. Blood. 2008. PMID: 18711001 Free PMC article.
-
Cooperation of p40(phox) with p47(phox) for Nox2-based NADPH oxidase activation during Fcγ receptor (FcγR)-mediated phagocytosis: mechanism for acquisition of p40(phox) phosphatidylinositol 3-phosphate (PI(3)P) binding.J Biol Chem. 2011 Nov 25;286(47):40693-705. doi: 10.1074/jbc.M111.237289. Epub 2011 Sep 28. J Biol Chem. 2011. PMID: 21956105 Free PMC article.
-
The domain organization of p67 phox, a protein required for activation of the superoxide-producing NADPH oxidase in phagocytes.J Innate Immun. 2009;1(6):543-55. doi: 10.1159/000235656. Epub 2009 Aug 18. J Innate Immun. 2009. PMID: 20375610
-
Role of the small GTPase Rac in p22phox-dependent NADPH oxidases.Biochimie. 2007 Sep;89(9):1133-44. doi: 10.1016/j.biochi.2007.05.003. Epub 2007 May 17. Biochimie. 2007. PMID: 17583407 Review.
-
Phagocytosis-coupled activation of the superoxide-producing phagocyte oxidase, a member of the NADPH oxidase (nox) family.Int J Hematol. 2006 Oct;84(3):193-8. doi: 10.1532/IJH97.06133. Int J Hematol. 2006. PMID: 17050190 Review.
Cited by
-
The role of IgG Fc receptors in antibody-dependent enhancement.Nat Rev Immunol. 2020 Oct;20(10):633-643. doi: 10.1038/s41577-020-00410-0. Epub 2020 Aug 11. Nat Rev Immunol. 2020. PMID: 32782358 Free PMC article. Review.
-
A conserved region between the TPR and activation domains of p67phox participates in activation of the phagocyte NADPH oxidase.J Biol Chem. 2010 Oct 8;285(41):31435-45. doi: 10.1074/jbc.M110.161166. Epub 2010 Aug 2. J Biol Chem. 2010. PMID: 20679349 Free PMC article.
-
A fluorescently tagged C-terminal fragment of p47phox detects NADPH oxidase dynamics during phagocytosis.Mol Biol Cell. 2009 Mar;20(5):1520-32. doi: 10.1091/mbc.e08-06-0620. Epub 2009 Jan 7. Mol Biol Cell. 2009. PMID: 19129478 Free PMC article.
-
Regulation of alveolar macrophage p40phox: hierarchy of activating kinases and their inhibition by PGE2.J Leukoc Biol. 2012 Jul;92(1):219-31. doi: 10.1189/jlb.1211590. Epub 2012 Apr 27. J Leukoc Biol. 2012. PMID: 22544939 Free PMC article.
-
Regulation of hydrogen peroxide release in circulating hemocytes of the planorbid snail Biomphalaria glabrata.Dev Comp Immunol. 2008;32(5):554-62. doi: 10.1016/j.dci.2007.09.001. Epub 2007 Oct 16. Dev Comp Immunol. 2008. PMID: 17981329 Free PMC article.
References
-
- Dinauer, M. 2003. The phagocyte system and disorders of granulopoiesis and granulocyte function. In Nathan and Oski's Hematology of Infancy and Childhood. D. Nathan, S. Orkin, D. Ginsburg, and A. Look, editors. W.B. Saunders Company, Philadelphia. 923–1010.
-
- Quinn, M.T., and K.A. Gauss. 2004. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 76:760–781. - PubMed
-
- Groemping, Y., K. Lapouge, S.J. Smerdon, and K. Rittinger. 2003. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 113:343–355. - PubMed
-
- Biberstine-Kinkade, K.J., L. Yu, and M.C. Dinauer. 1999. Mutagenesis of an arginine- and lysine-rich domain in the gp91phox subunit of the phagocyte NADPH-oxidase flavocytochrome b558. J. Biol. Chem. 274:10451–10457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

