Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1
- PMID: 16880258
- PMCID: PMC2118371
- DOI: 10.1084/jem.20060336
Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1
Abstract
Glucocorticoids (GCs), which are used in the treatment of immune-mediated inflammatory diseases, inhibit the expression of many inflammatory mediators. They can also induce the expression of dual specificity phosphatase 1 (DUSP1; otherwise known as mitogen-activated protein kinase [MAPK] phosphatase 1), which dephosphorylates and inactivates MAPKs. We investigated the role of DUSP1 in the antiinflammatory action of the GC dexamethasone (Dex). Dex-mediated inhibition of c-Jun N-terminal kinase and p38 MAPK was abrogated in DUSP1-/- mouse macrophages. Dex-mediated suppression of several proinflammatory genes (including tumor necrosis factor, cyclooxygenase 2, and interleukin 1alpha and 1beta) was impaired in DUSP1-/- mouse macrophages, whereas other proinflammatory genes were inhibited by Dex in a DUSP1-independent manner. In vivo antiinflammatory effects of Dex on zymosan-induced inflammation were impaired in DUSP1-/- mice. Therefore, the expression of DUSP1 is required for the inhibition of proinflammatory signaling pathways by Dex in mouse macrophages. Furthermore, DUSP1 contributes to the antiinflammatory effects of Dex in vitro and in vivo.
Figures


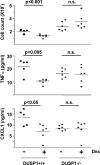
References
-
- Adcock, I.M., and S.J. Lane. 2003. Corticosteroid-insensitive asthma: molecular mechanisms. J. Endocrinol. 178:347–355. - PubMed
-
- Adcock, I.M., and G. Caramori. 2001. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 79:376–384. - PubMed
-
- Reichardt, H.M., K.H. Kaestner, J. Tuckermann, O. Kretz, O. Wessely, R. Bock, P. Gass, W. Schmid, P. Herrlich, P. Angel, and G. Schutz. 1998. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 93:531–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

