An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues
- PMID: 16880259
- PMCID: PMC2118376
- DOI: 10.1084/jem.20060376
An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues
Abstract
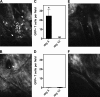
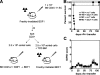
Transfer of T cells to freshly irradiated allogeneic recipients leads to their rapid recruitment to nonlymphoid tissues, where they induce graft-versus-host disease (GVHD). In contrast, when donor T cells are transferred to established mixed chimeras (MCs), GVHD is not induced despite a robust graft-versus-host (GVH) reaction that eliminates normal and malignant host hematopoietic cells. We demonstrate here that donor GVH-reactive T cells transferred to MCs or freshly irradiated mice undergo similar expansion and activation, with similar up-regulation of homing molecules required for entry to nonlymphoid tissues. Using dynamic two-photon in vivo microscopy, we show that these activated T cells do not enter GVHD target tissues in established MCs, contrary to the dogma that activated T cells inevitably traffic to nonlymphoid tissues. Instead, we show that the presence of inflammation within a nonlymphoid tissue is a prerequisite for the trafficking of activated T cells to that site. Our studies help to explain the paradox whereby GVH-reactive T cells can mediate graft-versus-leukemia responses without inducing GVHD in established MCs.
Figures



References
-
- Sprent, J., M. Schaefer, D. Lo, and R. Korngold. 1986. Functions of purified L3T4+ and Lyt-2+ cells in vitro and in vivo. Immunol. Rev. 91:195–218. - PubMed
-
- Shlomchik, W.D., M.S. Couzens, C.B. Tang, J. McNiff, M.E. Robert, J. Liu, M.J. Shlomchik, and S.G. Emerson. 1999. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 285:412–415. - PubMed
-
- Teshima, T., R. Ordemann, P. Reddy, S. Gagin, C. Liu, K.R. Cooke, and J.L. Ferrara. 2002. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat. Med. 8:575–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

