Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase
- PMID: 16880265
- PMCID: PMC2151529
- DOI: 10.1085/jgp.200609555
Fast adaptation in mouse olfactory sensory neurons does not require the activity of phosphodiesterase
Abstract
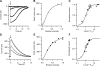
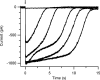
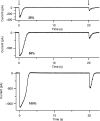
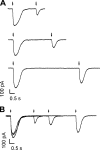
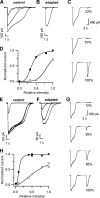
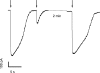
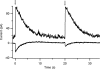
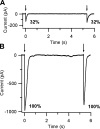
Vertebrate olfactory sensory neurons rapidly adapt to repetitive odorant stimuli. Previous studies have shown that the principal molecular mechanisms for odorant adaptation take place after the odorant-induced production of cAMP, and that one important mechanism is the negative feedback modulation by Ca2+-calmodulin (Ca2+-CaM) of the cyclic nucleotide-gated (CNG) channel. However, the physiological role of the Ca2+-dependent activity of phosphodiesterase (PDE) in adaptation has not been investigated yet. We used the whole-cell voltage-clamp technique to record currents in mouse olfactory sensory neurons elicited by photorelease of 8-Br-cAMP, an analogue of cAMP commonly used as a hydrolysis-resistant compound and known to be a potent agonist of the olfactory CNG channel. We measured currents in response to repetitive photoreleases of cAMP or of 8-Br-cAMP and we observed similar adaptation in response to the second stimulus. Control experiments were conducted in the presence of the PDE inhibitor IBMX, confirming that an increase in PDE activity was not involved in the response decrease. Since the total current activated by 8-Br-cAMP, as well as that physiologically induced by odorants, is composed not only of current carried by Na+ and Ca2+ through CNG channels, but also by a Ca2+-activated Cl- current, we performed control experiments in which the reversal potential of Cl- was set, by ion substitution, at the same value of the holding potential, -50 mV. Adaptation was measured also in these conditions of diminished Ca2+-activated Cl- current. Furthermore, by producing repetitive increases of ciliary's Ca2+ with flash photolysis of caged Ca2+, we showed that Ca2+-activated Cl- channels do not adapt and that there is no Cl- depletion in the cilia. All together, these results indicate that the activity of ciliary PDE is not required for fast adaptation to repetitive stimuli in mouse olfactory sensory neurons.
Figures
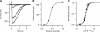

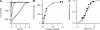
Comment in
-
The sweet smell of success: Conclusive evidence that cyclic AMP hydrolysis does not trigger fast adaptation in olfactory receptor cells.J Gen Physiol. 2006 Aug;128(2):149-51. doi: 10.1085/jgp.200609626. J Gen Physiol. 2006. PMID: 16880264 Free PMC article. No abstract available.
Similar articles
-
Temporal development of cyclic nucleotide-gated and Ca2+ -activated Cl- currents in isolated mouse olfactory sensory neurons.J Neurophysiol. 2007 Jul;98(1):153-60. doi: 10.1152/jn.00270.2007. Epub 2007 Apr 25. J Neurophysiol. 2007. PMID: 17460108
-
Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor.J Membr Biol. 1996 Jul;152(1):13-23. doi: 10.1007/s002329900081. J Membr Biol. 1996. PMID: 8660407
-
Mechanism of olfactory masking in the sensory cilia.J Gen Physiol. 2009 Jun;133(6):583-601. doi: 10.1085/jgp.200810085. Epub 2009 May 11. J Gen Physiol. 2009. PMID: 19433623 Free PMC article.
-
[Ion channels and action potentials in olfactory receptor cells].Nihon Shinkei Seishin Yakurigaku Zasshi. 2007 Nov;27(5-6):201-5. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007. PMID: 18154041 Review. Japanese.
-
Calcium signalling and regulation in olfactory neurons.Curr Opin Neurobiol. 1999 Aug;9(4):419-26. doi: 10.1016/S0959-4388(99)80063-4. Curr Opin Neurobiol. 1999. PMID: 10448159 Review.
Cited by
-
Calcium-activated chloride channels in the apical region of mouse vomeronasal sensory neurons.J Gen Physiol. 2012 Jul;140(1):3-15. doi: 10.1085/jgp.201210780. J Gen Physiol. 2012. PMID: 22732308 Free PMC article.
-
Odor-specific, olfactory marker protein-mediated sparsening of primary olfactory input to the brain after odor exposure.J Neurosci. 2013 Apr 10;33(15):6594-602. doi: 10.1523/JNEUROSCI.1442-12.2013. J Neurosci. 2013. PMID: 23575856 Free PMC article.
-
Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation.Neuron. 2008 May 8;58(3):374-86. doi: 10.1016/j.neuron.2008.02.029. Neuron. 2008. PMID: 18466748 Free PMC article.
-
The Ca2+-activated Cl- channel TMEM16B regulates action potential firing and axonal targeting in olfactory sensory neurons.J Gen Physiol. 2016 Oct;148(4):293-311. doi: 10.1085/jgp.201611622. Epub 2016 Sep 12. J Gen Physiol. 2016. PMID: 27619419 Free PMC article.
-
Role of plasma membrane calcium ATPases in calcium clearance from olfactory sensory neurons.Chem Senses. 2009 May;34(4):349-58. doi: 10.1093/chemse/bjp008. Epub 2009 Mar 20. Chem Senses. 2009. PMID: 19304763 Free PMC article.
References
-
- Balasubramanian, S., J.W. Lynch, and P.H. Barry. 1996. Calcium-dependent modulation of the agonist affinity of the mammalian olfactory cyclic nucleotide-gated channel by calmodulin and a novel endogenous factor. J. Membr. Biol. 152:13–23. - PubMed
-
- Beltman, J., D.E. Becker, E. Butt, G.S. Jensen, S.D. Rybalkin, B. Jastorff, and J.A. Beavo. 1995. Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Mol. Pharmacol. 47:330–339. - PubMed
-
- Boekhoff, I., C. Kroner, and H. Breer. 1996. Calcium controls second-messenger signalling in olfactory cilia. Cell. Signal. 8:167–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

