Interactions between heparan sulfate and proteins: the concept of specificity
- PMID: 16880267
- PMCID: PMC2064228
- DOI: 10.1083/jcb.200604035
Interactions between heparan sulfate and proteins: the concept of specificity
Abstract
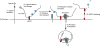
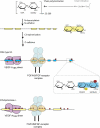
Proteoglycan (PG) coreceptors carry heparan sulfate (HS) chains that mediate interactions with growth factors, morphogens, and receptors. Thus, PGs modulate fundamental processes such as cell survival, division, adhesion, migration, and differentiation. This review summarizes recent biochemical and genetic information that sheds new light on the nature of HS-protein binding. Unexpectedly, many interactions appear to depend more on the overall organization of HS domains than on their fine structure.
Figures
References
-
- Ashikari-Hada, S., H. Habuchi, Y. Kariya, N. Itoh, A.H. Reddi, and K. Kimata. 2004. Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J. Biol. Chem. 279:12346–12354. - PubMed
-
- Ashikari-Hada, S., H. Habuchi, Y. Kariya, and K. Kimata. 2005. Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins. J. Biol. Chem. 280:31508–31515. - PubMed
-
- Bernfield, M., R. Kokenyesi, M. Kato, M.T. Hinkes, J. Spring, R.L. Gallo, and E.J. Lose. 1992. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu. Rev. Cell Biol. 8:365–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

