LMP4 regulates Tbx5 protein subcellular localization and activity
- PMID: 16880269
- PMCID: PMC2064230
- DOI: 10.1083/jcb.200511109
LMP4 regulates Tbx5 protein subcellular localization and activity
Abstract
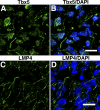
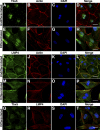
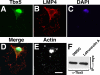
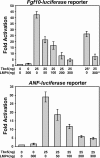
The limb- and heart-specific Tbx5 transcription factor coexpresses with and directly binds to the novel PDZ-LIM domain protein, LMP4. LMP4 is distributed in the cytoplasm associated with the actin cytoskeleton. In the presence of LMP4, Tbx5 shuttles dynamically between the nucleus and cytoplasm and, in a complex with LMP4, localizes to actin filaments. Nuclear and cytoplasmic Tbx5 distribution in developing chicken wings suggests the functional significance of the LMP4-Tbx5 interaction. In primary epicardial cells, we demonstrate that Tbx5 protein subcellular relocalization can be stimulated by external signals that induce cell differentiation. To test whether the relocalization from nuclear to cytoplasmic sites interferes with downstream gene expression, we used limb-specific Fgf10 and heart-specific Anf promoter-luciferase reporters and demonstrate that LMP4 acts as a repressor of Tbx5 activity. These studies reveal a previously unknown mechanism for Tbx transcription factor regulation in vertebrate limb and heart development and provide a better understanding of the molecular basis of hand/heart birth defects associated with Tbx5 mutations.
Figures


Similar articles
-
Differential regulation of Tbx5 protein expression and sub-cellular localization during heart development.Dev Biol. 2007 Feb 1;302(1):230-42. doi: 10.1016/j.ydbio.2006.09.023. Epub 2006 Sep 16. Dev Biol. 2007. PMID: 17045582 Free PMC article.
-
An evolutionarily conserved nuclear export signal facilitates cytoplasmic localization of the Tbx5 transcription factor.Mol Cell Biol. 2008 Mar;28(5):1553-64. doi: 10.1128/MCB.00935-07. Epub 2007 Dec 26. Mol Cell Biol. 2008. PMID: 18160705 Free PMC article.
-
Tbx5 and Tbx4 transcription factors interact with a new chicken PDZ-LIM protein in limb and heart development.Dev Biol. 2004 Sep 1;273(1):106-20. doi: 10.1016/j.ydbio.2004.05.024. Dev Biol. 2004. PMID: 15302601
-
Pdlim7 (LMP4) regulation of Tbx5 specifies zebrafish heart atrio-ventricular boundary and valve formation.Dev Biol. 2010 Jan 15;337(2):233-45. doi: 10.1016/j.ydbio.2009.10.039. Epub 2009 Nov 3. Dev Biol. 2010. PMID: 19895804 Free PMC article.
-
[A Literature Review on the Role of TBX5 in Expression and Progression of Lung Cancer: Current Perspectives].Zhongguo Fei Ai Za Zhi. 2020 Oct 20;23(10):883-888. doi: 10.3779/j.issn.1009-3419.2020.102.27. Epub 2020 Aug 19. Zhongguo Fei Ai Za Zhi. 2020. PMID: 32810974 Free PMC article. Review. Chinese.
Cited by
-
Negative Regulation of TGFβ Signaling by Stem Cell Antigen-1 Protects against Ischemic Acute Kidney Injury.PLoS One. 2015 Jun 8;10(6):e0129561. doi: 10.1371/journal.pone.0129561. eCollection 2015. PLoS One. 2015. PMID: 26053644 Free PMC article.
-
Soybean transcription factor ORFeome associated with drought resistance: a valuable resource to accelerate research on abiotic stress resistance.BMC Genomics. 2015 Aug 13;16(1):596. doi: 10.1186/s12864-015-1743-6. BMC Genomics. 2015. PMID: 26268547 Free PMC article.
-
Tbx5a and Tbx5b paralogues act in combination to control separate vectors of migration in the fin field of zebrafish.Dev Biol. 2022 Jan;481:201-214. doi: 10.1016/j.ydbio.2021.10.008. Epub 2021 Oct 28. Dev Biol. 2022. PMID: 34756968 Free PMC article.
-
T-box family of transcription factor-TBX5, insights in development and disease.Am J Transl Res. 2017 Feb 15;9(2):442-453. eCollection 2017. Am J Transl Res. 2017. PMID: 28337273 Free PMC article.
-
Loss of the cytoskeletal protein Pdlim7 predisposes mice to heart defects and hemostatic dysfunction.PLoS One. 2013 Nov 20;8(11):e80809. doi: 10.1371/journal.pone.0080809. eCollection 2013. PLoS One. 2013. PMID: 24278323 Free PMC article.
References
-
- Agarwal, P., J.N. Wylie, J. Galceran, O. Arkhitko, C. Li, C. Deng, R. Grosschedl, and B.G. Bruneau. 2003. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 130:623–633. - PubMed
-
- Ahn, D., M.J. Kourakis, L.A. Rohde, L.M. Silver, and R.K. Ho. 2002. T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature. 417:754–758. - PubMed
-
- Bach, I. 2000. The LIM domain: regulation by association. Mech. Dev. 91:5–17. - PubMed
-
- Boden, S.D., Y. Liu, G.A. Hair, J.A. Helms, D. Hu, M. Racine, M.S. Nanes, and L. Titus. 1998. LMP-1, a LIM-domain protein, mediates BMP-6 effects on bone formation. Endocrinology. 139:5125–5134. - PubMed
-
- Bruneau, B.G., M. Logan, N. Davis, T. Levi, C.J. Tabin, J.G. Seidman, and C.E. Seidman. 1999. Chamber-specific cardiac expression of Tbx5 and heart defects in Holt-Oram syndrome. Dev. Biol. 211:100–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

