Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells
- PMID: 16880387
- PMCID: PMC1567713
- DOI: 10.1073/pnas.0604850103
Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells
Abstract
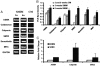
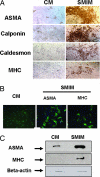
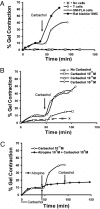
Smooth muscle is a major component of human tissues and is essential for the normal function of a multitude of organs including the intestine, urinary tract and the vascular system. The use of stem cells for cell-based tissue engineering and regeneration strategies represents a promising alternative for smooth muscle repair. For such strategies to succeed, a reliable source of smooth muscle precursor cells must be identified. Adipose tissue provides an abundant source of multipotent cells. In this study, the capacity of processed lipoaspirate (PLA) and adipose-derived stem cells to differentiate into phenotypic and functional smooth muscle cells was evaluated. To induce differentiation, PLA cells were cultured in smooth muscle differentiation medium. Smooth muscle differentiation of PLA cells induced genetic expression of all smooth muscle markers and further confirmed by increased protein expression of smooth muscle cell-specific alpha actin (ASMA), calponin, caldesmon, SM22, myosin heavy chain (MHC), and smoothelin. Clonal studies of adipose derived multipotent cells demonstrated differentiation of these cells into smooth muscle cells in addition to trilineage differentiation capacity. Importantly, smooth muscle-differentiated cells, but not their precursors, exhibit the functional ability to contract and relax in direct response to pharmacologic agents. In conclusion, adipose-derived cells have the potential to differentiate into functional smooth muscle cells and, thus, adipose tissue can be a useful source of cells for treatment of injured tissues where smooth muscle plays an important role.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Characterization of smooth muscle differentiation of purified human skeletal muscle-derived cells.J Cell Mol Med. 2011 Mar;15(3):587-92. doi: 10.1111/j.1582-4934.2010.01017.x. J Cell Mol Med. 2011. PMID: 20132408 Free PMC article.
-
Differentiation of adult stem cells into smooth muscle for vascular tissue engineering.J Surg Res. 2011 Jun 15;168(2):306-14. doi: 10.1016/j.jss.2009.08.001. Epub 2009 Sep 4. J Surg Res. 2011. PMID: 19959190 Free PMC article.
-
Differentiated markers in undifferentiated cells: expression of smooth muscle contractile proteins in multipotent bone marrow mesenchymal stem cells.Dev Growth Differ. 2013 Jun;55(5):591-605. doi: 10.1111/dgd.12052. Epub 2013 Apr 4. Dev Growth Differ. 2013. PMID: 23557080
-
Smooth muscle signalling pathways in health and disease.J Cell Mol Med. 2008 Dec;12(6A):2165-80. doi: 10.1111/j.1582-4934.2008.00552.x. J Cell Mol Med. 2008. PMID: 19120701 Free PMC article. Review.
-
[Caldesmon and calponin are proteins, participating in the regulation of myosin and actin interaction in nonmuscle and smooth muscle cells].Biokhimiia. 1991 Aug;56(8):1347-68. Biokhimiia. 1991. PMID: 1782263 Review. Russian.
Cited by
-
BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes.Lipids. 2015 Feb;50(2):111-20. doi: 10.1007/s11745-014-3981-9. Epub 2014 Dec 23. Lipids. 2015. PMID: 25534037 Free PMC article.
-
Externally Applied Static Magnetic Field Enhances Cardiac Retention and Functional Benefit of Magnetically Iron-Labeled Adipose-Derived Stem Cells in Infarcted Hearts.Stem Cells Transl Med. 2016 Oct;5(10):1380-1393. doi: 10.5966/sctm.2015-0220. Epub 2016 Jul 8. Stem Cells Transl Med. 2016. PMID: 27400797 Free PMC article.
-
The effect of low level laser irradiation on adult human adipose derived stem cells.Lasers Med Sci. 2008 Jul;23(3):277-82. doi: 10.1007/s10103-007-0479-1. Epub 2007 Aug 23. Lasers Med Sci. 2008. PMID: 17713825
-
The differentiation of human adipose-derived stem cells towards a urothelium-like phenotype in vitro and the dynamic temporal changes of related cytokines by both paracrine and autocrine signal regulation.PLoS One. 2014 Apr 21;9(4):e95583. doi: 10.1371/journal.pone.0095583. eCollection 2014. PLoS One. 2014. PMID: 24752317 Free PMC article.
-
Stem cell therapy for incontinence: where are we now? What is the realistic potential?Curr Urol Rep. 2011 Oct;12(5):336-44. doi: 10.1007/s11934-011-0210-4. Curr Urol Rep. 2011. PMID: 21842258 Free PMC article. Review.
References
-
- Lai J. Y., Yoon C. Y., Yoo J. J., Wulf T., Atala A. J. Urol. 2002;168:1853–1857. discussion, 1858. - PubMed
-
- Lin H. K., Cowan R., Moore P., Zhang Y., Yang Q., Peterson J. A., Jr., Tomasek J. J., Kropp B. P., Cheng E. Y. J. Urol. 2004;171:1348–1352. - PubMed
-
- Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D. M., et al. Nature. 2001;410:701–705. - PubMed
-
- Silva G. V., Litovsky S., Assad J. A., Sousa A. L., Martin B. J., Vela D., Coulter S. C., Lin J., Ober J., Vaughn W. K., et al. Circulation. 2005;111:150–156. - PubMed
-
- Religa P., Bojakowski K., Maksymowicz M., Bojakowska M., Sirsjo A., Gaciong Z., Olszewski W., Hedin U., Thyberg J. Transplantation. 2002;74:1310–1315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

