The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker
- PMID: 16880388
- PMCID: PMC1567710
- DOI: 10.1073/pnas.0604189103
The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker
Abstract
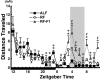
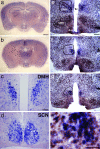
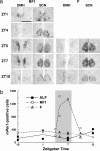
Temporal restriction of feeding can phase-shift behavioral and physiological circadian rhythms in mammals. These changes in biological rhythms are postulated to be brought about by a food-entrainable oscillator (FEO) that is independent of the suprachiasmatic nucleus. However, the neural substrates of FEO have remained elusive. Here, we carried out an unbiased search for mouse brain region(s) that exhibit a rhythmic expression of the Period genes in a feeding-entrainable manner. We found that the compact part of the dorsomedial hypothalamic nucleus (DMH) demonstrates a robust oscillation of mPer expression only under restricted feeding. The oscillation persisted for at least 2 days even when mice were given no food during the expected feeding period after the establishment of food-entrained behavioral rhythms. Moreover, refeeding after fasting rapidly induced a transient mPer expression in the same area of DMH. Taken in conjunction with recent findings (i) that behavioral expression of food-entrainable circadian rhythms is blocked by cell-specific lesions of DMH in rats and (ii) that DMH neurons directly project to orexin neurons in the lateral hypothalamus, which are essential for proper expression of food-entrained behavioral rhythms, the present study suggests that DMH plays a key role as a central FEO in the feeding-mediated regulation of circadian behaviors.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


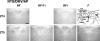
Similar articles
-
The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice.Eur J Neurosci. 2009 Apr;29(7):1447-60. doi: 10.1111/j.1460-9568.2009.06697.x. Epub 2009 Mar 23. Eur J Neurosci. 2009. PMID: 19519629
-
The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms.Nat Neurosci. 2006 Mar;9(3):398-407. doi: 10.1038/nn1651. Epub 2006 Feb 19. Nat Neurosci. 2006. PMID: 16491082
-
Circadian rhythms of PERIOD1 expression in the dorsomedial hypothalamic nucleus in the absence of entrained food-anticipatory activity rhythms in rats.Eur J Neurosci. 2009 Jun;29(11):2217-22. doi: 10.1111/j.1460-9568.2009.06766.x. Epub 2009 May 21. Eur J Neurosci. 2009. PMID: 19490091
-
Inducible clocks: living in an unpredictable world.Cold Spring Harb Symp Quant Biol. 2007;72:543-50. doi: 10.1101/sqb.2007.72.008. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419313 Review.
-
Neurobiology of food anticipatory circadian rhythms.Physiol Behav. 2011 Sep 26;104(4):535-45. doi: 10.1016/j.physbeh.2011.04.015. Epub 2011 Apr 20. Physiol Behav. 2011. PMID: 21527266 Review.
Cited by
-
Neurochemical and neuropharmacological aspects of circadian disruptions: an introduction to asynchronization.Curr Neuropharmacol. 2011 Jun;9(2):330-41. doi: 10.2174/157015911795596522. Curr Neuropharmacol. 2011. PMID: 22131941 Free PMC article.
-
GHS-R1a signaling in the DMH and VMH contributes to food anticipatory activity.Int J Obes (Lond). 2014 Apr;38(4):610-8. doi: 10.1038/ijo.2013.131. Epub 2013 Jul 25. Int J Obes (Lond). 2014. PMID: 23884084
-
High-Fat-Diet-Evoked Disruption of the Rat Dorsomedial Hypothalamic Clock Can Be Prevented by Restricted Nighttime Feeding.Nutrients. 2022 Nov 26;14(23):5034. doi: 10.3390/nu14235034. Nutrients. 2022. PMID: 36501063 Free PMC article.
-
Circadian acetylome reveals regulation of mitochondrial metabolic pathways.Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3339-44. doi: 10.1073/pnas.1217632110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341599 Free PMC article.
-
The central circadian timing system.Curr Opin Neurobiol. 2013 Oct;23(5):747-51. doi: 10.1016/j.conb.2013.04.004. Epub 2013 May 22. Curr Opin Neurobiol. 2013. PMID: 23706187 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

