Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes
- PMID: 16880396
- PMCID: PMC1567676
- DOI: 10.1073/pnas.0510306103
Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mu-cargo complexes
Abstract
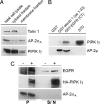
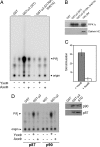
Phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P(2)] is an important factor for a variety of cellular functions ranging from cell signaling to actin cytoskeletal dynamics and endocytic membrane traffic. Here, we have identified the clathrin adaptor complex AP-2 as a regulator of phosphatidylinositol 4-phosphate 5-kinase (PIPK)-mediated PI(4,5)P(2) synthesis. AP-2 directly interacts with the kinase core domain of type I PIPK isozymes via its mu2-subunit in vitro and in native protein extracts. Endocytic cargo protein binding to mu2 leads to a potent stimulation of PIPK activity. These data thus identify a positive feedback loop consisting of endocytic cargo proteins, AP-2mu, and PIPK type I which may provide a specific pool of PI(4,5)P(2) dedicated to clathrin/AP-2-dependent receptor internalization.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
-
- Martin T. F. Curr. Opin. Cell Biol. 2001;13:493–499. - PubMed
-
- Yin H. L., Janmey P. A. Annu. Rev. Physiol. 2003;65:761–789. - PubMed
-
- Weernink P. A., Meletiadis K., Hommeltenberg S., Hinz M., Ishihara H., Schmidt M., Jakobs K. H. J. Biol. Chem. 2004;279:7840–7849. - PubMed
-
- Jost M., Simpson F., Kavran J. M., Lemmon M. A., Schmid S. L. Curr. Biol. 1998;8:1399–1402. - PubMed
-
- Donaldson J. G. J. Biol. Chem. 2003;278:41573–41576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

