Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines
- PMID: 16880402
- PMCID: PMC1567718
- DOI: 10.1073/pnas.0603571103
Manipulation of Arabidopsis fatty acid amide hydrolase expression modifies plant growth and sensitivity to N-acylethanolamines
Abstract
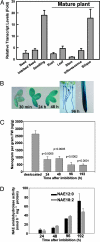
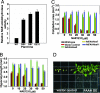
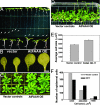
In vertebrates, the endocannabinoid signaling pathway is an important lipid regulatory pathway that modulates a variety of physiological and behavioral processes. N-Acylethanolamines (NAEs) comprise a group of fatty acid derivatives that function within this pathway, and their signaling activity is terminated by an enzyme called fatty acid amide hydrolase (FAAH), which hydrolyzes NAEs to ethanolamine and their corresponding free fatty acids. Bioinformatic approaches led to the identification of plant homologues of FAAH that are capable of hydrolyzing NAEs in vitro. To better understand the role of NAEs in plants, we identified T-DNA knockouts to Arabidopsis FAAH (AtFAAH; At5g64440) and generated plants overexpressing AtFAAH. Here we show that seeds of AtFAAH knockouts had elevated levels of endogenous NAEs, and seedling growth was hypersensitive to exogenously applied NAE. On the other hand, seeds and seedlings of AtFAAH overexpressors had lower endogenous NAE content, and seedlings were less sensitive to exogenous NAE. Moreover, AtFAAH overexpressors displayed enhanced seedling growth and increased cell size. AtFAAH expression and FAAH catalytic activity increased during seed germination and seedling growth, consistent with the timing of NAE depletion during seedling establishment. Collectively, our results show that AtFAAH is one, but not the only, modulator of endogenous NAE levels in plants, and that NAE depletion likely participates in the regulation of plant growth.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Mutations in Arabidopsis fatty acid amide hydrolase reveal that catalytic activity influences growth but not sensitivity to abscisic acid or pathogens.J Biol Chem. 2009 Dec 4;284(49):34065-74. doi: 10.1074/jbc.M109.059022. Epub 2009 Sep 30. J Biol Chem. 2009. PMID: 19801664 Free PMC article.
-
Overexpression of Fatty Acid Amide Hydrolase Induces Early Flowering in Arabidopsis thaliana.Front Plant Sci. 2012 Feb 20;3:32. doi: 10.3389/fpls.2012.00032. eCollection 2012. Front Plant Sci. 2012. PMID: 22645580 Free PMC article.
-
A chemical genetic screen uncovers a small molecule enhancer of the N-acylethanolamine degrading enzyme, fatty acid amide hydrolase, in Arabidopsis.Sci Rep. 2017 Jan 23;7:41121. doi: 10.1038/srep41121. Sci Rep. 2017. PMID: 28112243 Free PMC article.
-
The N-acylethanolamine-mediated regulatory pathway in plants.Chem Biodivers. 2007 Aug;4(8):1933-55. doi: 10.1002/cbdv.200790161. Chem Biodivers. 2007. PMID: 17712835 Review.
-
N-Acylethanolamines: lipid metabolites with functions in plant growth and development.Plant J. 2014 Aug;79(4):568-83. doi: 10.1111/tpj.12427. Epub 2014 Feb 25. Plant J. 2014. PMID: 24397856 Review.
Cited by
-
Ethanolamide oxylipins of linolenic acid can negatively regulate Arabidopsis seedling development.Plant Cell. 2013 Oct;25(10):3824-40. doi: 10.1105/tpc.113.119024. Epub 2013 Oct 22. Plant Cell. 2013. PMID: 24151297 Free PMC article.
-
Plants and Small Molecules: An Up-and-Coming Synergy.Plants (Basel). 2023 Apr 21;12(8):1729. doi: 10.3390/plants12081729. Plants (Basel). 2023. PMID: 37111951 Free PMC article. Review.
-
An endocannabinoid catabolic enzyme FAAH and its paralogs in an early land plant reveal evolutionary and functional relationship with eukaryotic orthologs.Sci Rep. 2020 Feb 20;10(1):3115. doi: 10.1038/s41598-020-59948-7. Sci Rep. 2020. PMID: 32080293 Free PMC article.
-
Synthesis of phenoxyacyl-ethanolamides and their effects on fatty acid amide hydrolase activity.J Biol Chem. 2014 Mar 28;289(13):9340-51. doi: 10.1074/jbc.M113.533315. Epub 2014 Feb 20. J Biol Chem. 2014. PMID: 24558037 Free PMC article.
-
N-acylethanolamine (NAE) inhibits growth in Arabidopsis thaliana seedlings via ABI3-dependent and -independent pathways.Plant Signal Behav. 2011 May;6(5):671-9. doi: 10.4161/psb.6.5.14704. Epub 2011 May 1. Plant Signal Behav. 2011. PMID: 21633189 Free PMC article.
References
-
- Devane W. A., Hanus L., Breuer A., Pertwee R. G., Stevenson L. A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Science. 1992;258:1946–1949. - PubMed
-
- Chapman K. D. Chem. Phys. Lipids. 2000;108:221–230. - PubMed
-
- Chapman K. D. Prog. Lipid Res. 2004;43:302–327. - PubMed
-
- Blancaflor E. B., Chapman K. D. In: Communication in Plants–Neuronal Aspects of Plant Life. Baluška F., Mancuso S., Volkmann D., editors. Heidelberg, Germany: Springer; 2006. pp. 205–219.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

