15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis
- PMID: 16880406
- PMCID: PMC1567703
- DOI: 10.1073/pnas.0603235103
15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis
Abstract
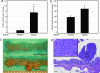
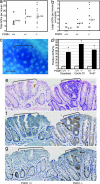
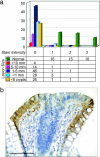
15-Hydroxyprostaglandin dehydrogenase (15-PGDH) is a prostaglandin-degrading enzyme that is highly expressed in normal colon mucosa but is ubiquitously lost in human colon cancers. Herein, we demonstrate that 15-PGDH is active in vivo as a highly potent suppressor of colon neoplasia development and acts in the colon as a required physiologic antagonist of the prostaglandin-synthesizing activity of the cyclooxygenase 2 (COX-2) oncogene. We first show that 15-PGDH gene knockout induces a marked 7.6-fold increase in colon tumors arising in the Min (multiple intestinal neoplasia) mouse model. Furthermore, 15-PGDH gene knockout abrogates the normal resistance of C57BL/6J mice to colon tumor induction by the carcinogen azoxymethane (AOM), conferring susceptibility to AOM-induced adenomas and carcinomas in situ. Susceptibility to AOM-induced tumorigenesis is mediated by a marked induction of dysplasia, proliferation, and cyclin D1 expression throughout microscopic aberrant crypt foci arising in 15-PGDH null colons and is concomitant with a doubling of prostaglandin E(2) in 15-PGDH null colonic mucosa. A parallel role for 15-PGDH loss in promoting the earliest steps of colon neoplasia in humans is supported by our finding of a universal loss of 15-PGDH expression in microscopic colon adenomas recovered from patients with familial adenomatous polyposis, including adenomas as small as a single crypt. These models thus delineate the in vivo significance of 15-PGDH-mediated negative regulation of the COX-2 pathway and moreover reveal the particular importance of 15-PGDH in opposing the neoplastic progression of colonic aberrant crypt foci.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
Sulindac reversal of 15-PGDH-mediated resistance to colon tumor chemoprevention with NSAIDs.Carcinogenesis. 2015 Feb;36(2):291-8. doi: 10.1093/carcin/bgu241. Epub 2014 Dec 10. Carcinogenesis. 2015. PMID: 25503930 Free PMC article.
-
15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers.Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17468-73. doi: 10.1073/pnas.0406142101. Epub 2004 Dec 1. Proc Natl Acad Sci U S A. 2004. PMID: 15574495 Free PMC article.
-
15-Hydroxyprostaglandin dehydrogenase inactivation as a mechanism of resistance to celecoxib chemoprevention of colon tumors.Proc Natl Acad Sci U S A. 2009 Jun 9;106(23):9409-13. doi: 10.1073/pnas.0902367106. Epub 2009 May 22. Proc Natl Acad Sci U S A. 2009. PMID: 19470469 Free PMC article. Clinical Trial.
-
Regulation of 15-hydroxyprostaglandin dehydrogenase (15-PGDH) by non-steroidal anti-inflammatory drugs (NSAIDs).Prostaglandins Other Lipid Mediat. 2011 Nov;96(1-4):37-40. doi: 10.1016/j.prostaglandins.2011.06.005. Epub 2011 Jul 6. Prostaglandins Other Lipid Mediat. 2011. PMID: 21763448 Review.
-
Prostaglandin catabolic enzymes as tumor suppressors.Cancer Metastasis Rev. 2011 Dec;30(3-4):409-17. doi: 10.1007/s10555-011-9314-z. Cancer Metastasis Rev. 2011. PMID: 22020925 Review.
Cited by
-
Tumour suppressor 15-hydroxyprostaglandin dehydrogenase induces differentiation in colon cancer via GLI1 inhibition.Oncogenesis. 2020 Aug 19;9(8):74. doi: 10.1038/s41389-020-00256-0. Oncogenesis. 2020. PMID: 32814764 Free PMC article.
-
Reduction of 15-hydroxyprostaglandin dehydrogenase expression is an independent predictor of poor survival associated with enhanced cell proliferation in gastric adenocarcinoma.Cancer Sci. 2010 Feb;101(2):550-8. doi: 10.1111/j.1349-7006.2009.01390.x. Epub 2009 Oct 10. Cancer Sci. 2010. PMID: 19917058 Free PMC article.
-
15-PGDH regulates hematopoietic and gastrointestinal fitness during aging.PLoS One. 2022 May 19;17(5):e0268787. doi: 10.1371/journal.pone.0268787. eCollection 2022. PLoS One. 2022. PMID: 35587945 Free PMC article.
-
Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis.Cancer Sci. 2011 Apr;102(4):713-9. doi: 10.1111/j.1349-7006.2011.01847.x. Epub 2011 Feb 2. Cancer Sci. 2011. PMID: 21205091 Free PMC article.
-
Mouse models for the study of colon carcinogenesis.Carcinogenesis. 2009 Feb;30(2):183-96. doi: 10.1093/carcin/bgn267. Epub 2008 Nov 26. Carcinogenesis. 2009. PMID: 19037092 Free PMC article. Review.
References
-
- Tai H. H., Ensor C. M., Tong M., Zhou H., Yan F. Prostaglandins Other Lipid Mediat. 2002;68–69:483–493. - PubMed
-
- Brown J. R., DuBois R. N. J. Clin. Oncol. 2005;23:2840–2855. - PubMed
-
- Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. Cell. 1996;87:803–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

