Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta
- PMID: 16880506
- PMCID: PMC1592798
- DOI: 10.1128/MCB.00383-06
Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase beta
Abstract
AMP-activated protein kinase (AMPK) is a sensor of cellular energy state in response to metabolic stress and other regulatory signals. AMPK is controlled by upstream kinases which have recently been identified as LKB1 or Ca2+/calmodulin-dependent protein kinase kinase beta (CaMKKbeta). Our study of human endothelial cells shows that AMPK is activated by thrombin through a Ca2+-dependent mechanism involving the thrombin receptor protease-activated receptor 1 and Gq-protein-mediated phospholipase C activation. Inhibition of CaMKK with STO-609 or downregulation of CaMKKbeta using RNA interference decreased thrombin-induced AMPK activation significantly, indicating that CaMKKbeta was the responsible AMPK kinase. In contrast, downregulation of LKB1 did not affect thrombin-induced AMPK activation but abolished phosphorylation of AMPK with 5-aminoimidazole-4-carboxamide ribonucleoside. Thrombin stimulation led to phosphorylation of acetyl coenzyme A carboxylase (ACC) and endothelial nitric oxide synthase (eNOS), two downstream targets of AMPK. Inhibition or downregulation of CaMKKbeta or AMPK abolished phosphorylation of ACC in response to thrombin but had no effect on eNOS phosphorylation, indicating that thrombin-stimulated phosphorylation of eNOS is not mediated by AMPK. Our results underline the role of Ca2+ as a regulator of AMPK activation in response to a physiologic stimulation. We also demonstrate that endothelial cells possess two pathways to activate AMPK, one Ca2+/CaMKKbeta dependent and one AMP/LKB1 dependent.
Figures
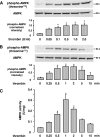
 , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.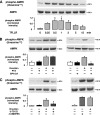
 , P < 0.05.
, P < 0.05.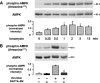
 , P < 0.05.
, P < 0.05.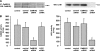
 , P < 0.05 versus control siRNA.
, P < 0.05 versus control siRNA.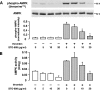
 , P < 0.05 versus untreated thrombin-stimulated controls.
, P < 0.05 versus untreated thrombin-stimulated controls.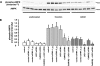
 , P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.
, P < 0.05 versus samples pretreated with control siRNA and stimulated with thrombin or AICAR.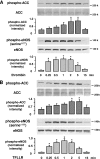
 , P < 0.05 versus untreated controls.
, P < 0.05 versus untreated controls.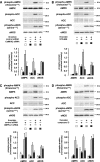
 , P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).
, P < 0.05 versus thrombin-stimulated samples pretreated with control siRNA (A and C) or solvent (B and D).Similar articles
-
eNOS activation mediated by AMPK after stimulation of endothelial cells with histamine or thrombin is dependent on LKB1.Biochim Biophys Acta. 2011 Feb;1813(2):322-31. doi: 10.1016/j.bbamcr.2010.12.001. Epub 2010 Dec 9. Biochim Biophys Acta. 2011. PMID: 21145922
-
Mechanism of thrombin mediated eNOS phosphorylation in endothelial cells is dependent on ATP levels after stimulation.Biochim Biophys Acta. 2008 Oct;1783(10):1893-902. doi: 10.1016/j.bbamcr.2008.07.003. Epub 2008 Jul 18. Biochim Biophys Acta. 2008. PMID: 18687367
-
Estrogen Activates AMP-Activated Protein Kinase in Human Endothelial Cells via ERβ/Ca(2+)/Calmodulin-Dependent Protein Kinase Kinase β Pathway.Cell Biochem Biophys. 2015 Jul;72(3):701-7. doi: 10.1007/s12013-015-0521-z. Cell Biochem Biophys. 2015. PMID: 25616441
-
AMP-activated protein kinase in metabolic control and insulin signaling.Circ Res. 2007 Feb 16;100(3):328-41. doi: 10.1161/01.RES.0000256090.42690.05. Circ Res. 2007. PMID: 17307971 Review.
-
AMP-activated protein kinase: implications on ischemic diseases.BMB Rep. 2012 Sep;45(9):489-95. doi: 10.5483/bmbrep.2012.45.9.169. BMB Rep. 2012. PMID: 23010169 Review.
Cited by
-
AMP-activated protein kinase regulates L-arginine mediated cellular responses.Nutr Metab (Lond). 2013 May 29;10(1):40. doi: 10.1186/1743-7075-10-40. Nutr Metab (Lond). 2013. PMID: 23718875 Free PMC article.
-
Emerging Role of cAMP/AMPK Signaling.Cells. 2022 Jan 17;11(2):308. doi: 10.3390/cells11020308. Cells. 2022. PMID: 35053423 Free PMC article. Review.
-
Beta-arrestin inhibits CAMKKbeta-dependent AMPK activation downstream of protease-activated-receptor-2.BMC Biochem. 2010 Sep 21;11:36. doi: 10.1186/1471-2091-11-36. BMC Biochem. 2010. PMID: 20858278 Free PMC article.
-
AMPK: a target for drugs and natural products with effects on both diabetes and cancer.Diabetes. 2013 Jul;62(7):2164-72. doi: 10.2337/db13-0368. Diabetes. 2013. PMID: 23801715 Free PMC article. Review.
-
Molecular Pathways: Is AMPK a Friend or a Foe in Cancer?Clin Cancer Res. 2015 Sep 1;21(17):3836-40. doi: 10.1158/1078-0432.CCR-14-3300. Epub 2015 Jul 7. Clin Cancer Res. 2015. PMID: 26152739 Free PMC article. Review.
References
-
- Aley, P. K., K. E. Porter, J. P. Boyle, P. J. Kemp, and C. Peers. 2005. Hypoxic modulation of Ca2+ signaling in human venous endothelial cells. Multiple roles for reactive oxygen species. J. Biol. Chem. 280:13349-13354. - PubMed
-
- Reference deleted.
-
- Bogatcheva, N. V., J. G. Garcia, and A. D. Verin. 2002. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry (Moscow) 67:75-84. - PubMed
-
- Borbiev, T., A. D. Verin, S. Shi, F. Liu, and J. G. Garcia. 2001. Regulation of endothelial cell barrier function by calcium/calmodulin-dependent protein kinase II. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L983-L990. - PubMed
-
- Cacicedo, J. M., N. Yagihashi, J. F. Keaney, Jr., N. B. Ruderman, and Y. Ido. 2004. AMPK inhibits fatty acid-induced increases in NF-κB transactivation in cultured human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 324:1204-1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
