Negative regulation of interleukin-2 and p38 mitogen-activated protein kinase during T-cell activation by the adaptor ALX
- PMID: 16880512
- PMCID: PMC1592799
- DOI: 10.1128/MCB.02067-05
Negative regulation of interleukin-2 and p38 mitogen-activated protein kinase during T-cell activation by the adaptor ALX
Abstract
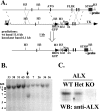
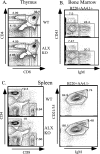
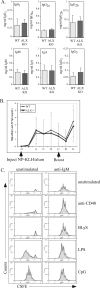
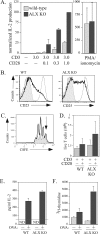
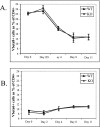
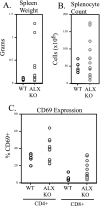
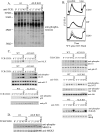
Activation of naïve T cells requires synergistic signals produced by the T-cell receptor (TCR) and by CD28. We previously identified the novel adaptor ALX, which, upon overexpression in Jurkat T cells, inhibited activation of the interleukin-2 (IL-2) promoter by TCR/CD28, suggesting that it is a negative regulator of T-cell activation. To further understand the physiological role of ALX, ALX-deficient mice were generated. Purified T cells from ALX-deficient mice demonstrated increased IL-2 production, CD25 expression, and proliferation in response to TCR/CD28 stimulation. Enhanced IL-2 production and proliferation were also observed when ALX-deficient mice were primed in vivo with ovalbumin-complete Freund's adjuvant and then restimulated ex vivo. Consistent with our initial overexpression studies, these data demonstrate that ALX is a negative regulator of T-cell activation. While TCR/CD28-mediated activations of phosphotyrosine induction, extracellular signal-regulated kinase 1/2, Jun N-terminal protein kinase, IkappaB kinase alpha/beta, and Akt were unaltered, constitutive activation of p38 mitogen-activated protein kinase and its upstream regulators MKK3/6 were observed for ALX-deficient splenocytes. The phenotype of ALX-deficient mice resembled the phenotype of those deficient in the transmembrane adaptor LAX, and an association between ALX and LAX proteins was demonstrated. These results suggest that ALX, in association with LAX, negatively regulates T-cell activation through inhibition of p38.
Figures

Similar articles
-
The related adaptors, adaptor in lymphocytes of unknown function X and Rlk/Itk-binding protein, have nonredundant functions in lymphocytes.J Immunol. 2007 Aug 1;179(3):1768-75. doi: 10.4049/jimmunol.179.3.1768. J Immunol. 2007. PMID: 17641043
-
Negative regulation of TCR signaling by linker for activation of X cells via phosphotyrosine-dependent and -independent mechanisms.J Immunol. 2008 Nov 15;181(10):7055-61. doi: 10.4049/jimmunol.181.10.7055. J Immunol. 2008. PMID: 18981125 Free PMC article.
-
Dual-specificity phosphatase 14 (DUSP14/MKP6) negatively regulates TCR signaling by inhibiting TAB1 activation.J Immunol. 2014 Feb 15;192(4):1547-57. doi: 10.4049/jimmunol.1300989. Epub 2014 Jan 8. J Immunol. 2014. PMID: 24403530
-
Control of lymphocyte development and activation by negative regulatory transmembrane adapter proteins.Immunol Rev. 2008 Aug;224:215-28. doi: 10.1111/j.1600-065X.2008.00656.x. Immunol Rev. 2008. PMID: 18759929 Review.
-
The T-cell-specific adapter protein family: TSAd, ALX, and SH2D4A/SH2D4B.Immunol Rev. 2009 Nov;232(1):240-54. doi: 10.1111/j.1600-065X.2009.00829.x. Immunol Rev. 2009. PMID: 19909368 Review.
Cited by
-
Alternative ZAP70-p38 signals prime a classical p38 pathway through LAT and SOS to support regulatory T cell differentiation.Sci Signal. 2019 Jul 23;12(591):eaao0736. doi: 10.1126/scisignal.aao0736. Sci Signal. 2019. PMID: 31337738 Free PMC article.
-
Adapters in the organization of mast cell signaling.Immunol Rev. 2009 Nov;232(1):195-217. doi: 10.1111/j.1600-065X.2009.00834.x. Immunol Rev. 2009. PMID: 19909365 Free PMC article. Review.
-
Phosphorylation at serine 318 is not required for inhibition of T cell activation by ALX.Biochem Biophys Res Commun. 2010 Jun 11;396(4):994-8. doi: 10.1016/j.bbrc.2010.05.043. Epub 2010 May 13. Biochem Biophys Res Commun. 2010. PMID: 20471366 Free PMC article.
-
Differential expression of the adaptor protein HSH2 controls the quantitative and qualitative nature of the humoral response.J Immunol. 2011 Oct 1;187(7):3565-77. doi: 10.4049/jimmunol.1101534. Epub 2011 Aug 26. J Immunol. 2011. PMID: 21873522 Free PMC article.
-
Host responses to live-attenuated Venezuelan equine encephalitis virus (TC-83): comparison of naïve, vaccine responder and nonresponder to TC-83 challenge in human peripheral blood mononuclear cells.Hum Vaccin Immunother. 2012 Aug;8(8):1053-65. doi: 10.4161/hv.20300. Epub 2012 Aug 1. Hum Vaccin Immunother. 2012. PMID: 22617845 Free PMC article.
References
-
- Chambers, C. A., and J. P. Allison. 1997. Co-stimulation in T cell responses. Curr. Opin. Immunol. 9:396-404. - PubMed
-
- Chen, C. Y., F. Del Gatto-Konczak, Z. Wu, and M. Karin. 1998. Stabilization of interleukin-2 mRNA by the c-Jun NH2-terminal kinase pathway. Science 280:1945-1949. - PubMed
-
- Choi, Y. B., C. K. Kim, and Y. Yun. 1999. Lad, an adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation. J. Immunol. 163:5242-5249. - PubMed
-
- Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. - PubMed
-
- Espel, E. 2005. The role of the AU-rich elements of mRNAs in controlling translation. Semin. Cell Dev. Biol. 16:59-67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
