Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells
- PMID: 16880514
- PMCID: PMC1592795
- DOI: 10.1128/MCB.02354-05
Wnt-5a/Ca2+-induced NFAT activity is counteracted by Wnt-5a/Yes-Cdc42-casein kinase 1alpha signaling in human mammary epithelial cells
Abstract
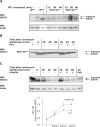
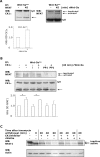
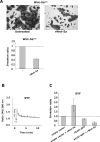
Wnt-5a has been shown to influence the metastatic behavior of human breast cancer cells, and the loss of Wnt-5a expression is associated with metastatic disease. We show here that NFAT1, a transcription factor connected with breast cancer metastasis, is activated by Wnt-5a through a Ca2+ signaling pathway in human breast epithelial cells. This activation was simultaneously counteracted by a Wnt-5a-induced Yes/Cdc42 signaling pathway. The observation that inhibition of the Wnt-5a/Yes/Cdc42 signal prolonged the duration of ionomycin-induced NFAT1 activation revealed the general importance of this pathway. The Wnt-5a-induced inhibition of NFAT1 did not require glycogen synthase kinase 3beta, JNK, or Pak1 activity or modulation of the cytoskeleton. Instead, we observed that Wnt-5a induced a complex formation of NFAT1/casein kinase 1alpha, even upon treatment with ionomycin, which was blocked upon inhibition of the Wnt-5a/Yes/Cdc42 signaling pathway. Our results explain why Wnt-5a/Ca2+-induced NFAT activity is hard to detect and suggest a novel mechanism by which Wnt-5a can suppress tumor-specific, agonist-induced NFAT activity and thus the metastatic behavior of breast cancer cells.
Figures





References
-
- Adam, L., R. Vadlamudi, M. Mandal, J. Chernoff, and R. Kumar. 2000. Regulation of microfilament reorganization and invasiveness of breast cancer cells by kinase dead p21-activated kinase-1. J. Biol. Chem. 275:12041-12050. - PubMed
-
- Aramburu, J., M. B. Yaffe, C. Lopez-Rodriguez, L. C. Cantley, P. G. Hogan, and A. Rao. 1999. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science 285:2129-2133. - PubMed
-
- Beals, C. R., C. M. Sheridan, C. W. Turck, P. Gardner, and G. R. Crabtree. 1997. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930-1934. - PubMed
-
- Chow, C. W., M. Rincon, J. Cavanagh, M. Dickens, and R. J. Davis. 1997. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278:1638-1641. - PubMed
-
- Crabtree, G. R., and E. N. Olson. 2002. NFAT signaling: choreographing the social lives of cells. Cell 109(Suppl.):S67-S79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
