Systemically administered trefoil factors are secreted into the gastric lumen and increase the viscosity of gastric contents
- PMID: 16880764
- PMCID: PMC1629411
- DOI: 10.1038/sj.bjp.0706840
Systemically administered trefoil factors are secreted into the gastric lumen and increase the viscosity of gastric contents
Abstract
Background and purpose: Trefoil factors (TFFs) secreted by mucus-producing cells are essential for the defence of the gastrointestinal mucosa. TFFs probably influence the viscoelastic properties of mucus, but this has not been demonstrated in vivo. We therefore studied the gastric secretion of systemically administered TFF2 and TFF3, and their influence on the viscosity of the secretions.
Experimental approach: Mice and rats under general anaesthesia were injected intravenously with human (h) TFF2, hTFF3 (5 mg kg(-1) to mice and 25 mg kg(-1) to rats), murine (m) (125)I-TFF3, or (125)I-hTFF3 (300,000 cpm, mice only). The appearance of TFFs in the gastric mucosa and luminal secretions was analysed by autoradiography, gamma-counting, and ELISA, and the viscosity by rheometry.
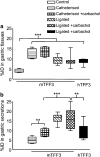
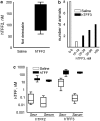
Key results: (125)I-mTFF3 and (125)I-hTFF3 were taken up by secretory cells of the gastrointestinal tract and detected at the gastric mucosal surface 15 min after injection. Stressing the stomach by carbachol (3.5 microg kg(-1)) and pyloric ligation significantly increased the uptake. Injected hTFF2, hTFF3, and mTFF3 were retrieved from the gastric contents after 4 h. In rats, an approximately seven-fold increase in the viscosity was detected after injection of TFF2 compared to the controls, whereas TFF3 did not increase the viscosity. In mice, TFF2 increased the viscosity approximately 4-fold.
Conclusions: These data indicate that systemically administered TFFs are transferred to the gastric lumen in a biologically active form.
Figures


Similar articles
-
Pharmacokinetics of trefoil peptides and their stability in gastrointestinal contents.Peptides. 2007 Jun;28(6):1197-206. doi: 10.1016/j.peptides.2007.03.016. Epub 2007 Mar 31. Peptides. 2007. PMID: 17466412
-
Injected TFF1 and TFF3 bind to TFF2-immunoreactive cells in the gastrointestinal tract in rats.Regul Pept. 2003 Sep 15;115(2):91-9. doi: 10.1016/s0167-0115(03)00145-9. Regul Pept. 2003. PMID: 12972324
-
Luminal and parenteral TFF2 and TFF3 dimer and monomer in two models of experimental colitis in the rat.Regul Pept. 2005 Mar 30;126(3):163-71. doi: 10.1016/j.regpep.2004.09.007. Regul Pept. 2005. PMID: 15664663
-
Trefoil factors and human gastric cancer (review).Int J Mol Med. 2003 Jul;12(1):3-9. Int J Mol Med. 2003. PMID: 12792801 Review.
-
Subcellular distribution of peptides associated with gastric mucosal healing and neoplasia.Microsc Res Tech. 1995 Jun 15;31(3):234-47. doi: 10.1002/jemt.1070310307. Microsc Res Tech. 1995. PMID: 7670162 Review.
Cited by
-
Trefoil factors in inflammatory bowel disease.World J Gastroenterol. 2014 Mar 28;20(12):3223-30. doi: 10.3748/wjg.v20.i12.3223. World J Gastroenterol. 2014. PMID: 24696606 Free PMC article. Review.
-
Excess Secretion of Gel-Forming Mucins and Associated Innate Defense Proteins with Defective Mucin Un-Packaging Underpin Gallbladder Mucocele Formation in Dogs.PLoS One. 2015 Sep 28;10(9):e0138988. doi: 10.1371/journal.pone.0138988. eCollection 2015. PLoS One. 2015. PMID: 26414376 Free PMC article.
-
The TFF Peptides xP1 and xP4 Appear in Distinctive Forms in the Xenopus laevis Gastric Mucosa: Indications for Different Protective Functions.Int J Mol Sci. 2019 Nov 30;20(23):6052. doi: 10.3390/ijms20236052. Int J Mol Sci. 2019. PMID: 31801293 Free PMC article.
-
Pathological and therapeutic roles of bioactive peptide trefoil factor 3 in diverse diseases: recent progress and perspective.Cell Death Dis. 2022 Jan 17;13(1):62. doi: 10.1038/s41419-022-04504-6. Cell Death Dis. 2022. PMID: 35039476 Free PMC article. Review.
-
Decreased expression of TFF2 and decreased αGlcNAc glycosylation are malignant biomarkers of pyloric gland adenoma of the duodenum.Sci Rep. 2023 Dec 8;13(1):21641. doi: 10.1038/s41598-023-49040-1. Sci Rep. 2023. PMID: 38062108 Free PMC article.
References
-
- Babyatsky MW, Debeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. - PubMed
-
- Chinery R, Cox HM. Immunoprecipitation and characterization of a binding protein specific for the peptide, intestinal trefoil factor. Peptides. 1995a;16:749–755. - PubMed
-
- Chinery R, Playford RJ. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci (Lond) 1995b;88:401–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

