Matrix metalloproteinases contribute to endotoxin and interleukin-1beta induced vascular dysfunction
- PMID: 16880766
- PMCID: PMC1629401
- DOI: 10.1038/sj.bjp.0706823
Matrix metalloproteinases contribute to endotoxin and interleukin-1beta induced vascular dysfunction
Abstract
Background and purpose: The acute vascular inflammatory dysfunction associated with endotoxaemia may reflect an imbalance between matrix metalloproteinases (MMPs) and their natural inhibitors (TIMPs), induced by the endotoxin. This possibility was tested in rat aortic tissue.
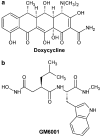
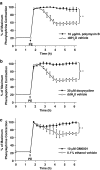
Experimental approaches: Tone induced by phenylephrine in aortic rings was measured after exposure in vitro to ambient lipopolysaccharide (LPS) or the proinflammatory cytokine interleukin-1beta (IL-1beta) for 6h, with or without MMP inhibitors (doxycycline or GM6001). Gelatinase and MMP activities, TIMP proteins and contractility were measured in aortae taken from rats 6h after receiving LPS in vivo.
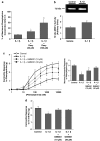
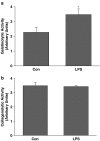
Key results: Inhibition of MMP prevented the loss of phenylephrine-induced tone in aortic rings after LPS or IL-1beta. IL-1beta also increased release of MMP-2 activity from aortic tissue. In aortae exposed in vivo to LPS, net gelatinase, MMP-9 activities and TIMP-1 protein levels were increased, whereas TIMP-4 was reduced. These aortae were hypocontractile to both phenylephrine and KCl. Hypocontractility was partially reversed by doxycycline ex vivo.
Conclusions and implications: MMP inhibitors ameliorate vascular hyporeactivity induced by either LPS or IL-1beta in vitro. LPS in vivo alters the balance between MMPs and TIMPs, contributing to vascular dysfunction which is partially reversed by MMP inhibitors. Vascular MMPs are activated as a result of LPS or IL-1beta-induced stress and contribute to the hyporeactivity of blood vessels to vasoconstrictors.
Figures


Similar articles
-
Endothelial dependence of matrix metalloproteinase-mediated vascular hyporeactivity caused by lipopolysaccharide.Eur J Pharmacol. 2008 Mar 17;582(1-3):116-22. doi: 10.1016/j.ejphar.2007.12.019. Epub 2007 Dec 28. Eur J Pharmacol. 2008. PMID: 18242597
-
Inhibition of matrix metalloproteinase activity in vivo protects against vascular hyporeactivity in endotoxemia.Am J Physiol Heart Circ Physiol. 2010 Jan;298(1):H45-51. doi: 10.1152/ajpheart.00273.2009. Epub 2009 Oct 16. Am J Physiol Heart Circ Physiol. 2010. PMID: 19837953
-
Matrix metalloproteinase-2 proteolysis of calponin-1 contributes to vascular hypocontractility in endotoxemic rats.Arterioscler Thromb Vasc Biol. 2012 Mar;32(3):662-8. doi: 10.1161/ATVBAHA.111.242685. Epub 2011 Dec 22. Arterioscler Thromb Vasc Biol. 2012. PMID: 22199370
-
Matrix metalloproteinases and their inhibitors.Anticancer Res. 1999 Mar-Apr;19(2C):1589-92. Anticancer Res. 1999. PMID: 10365151 Review.
-
Matrix metalloproteinases and periodontal diseases.Oral Dis. 2014 Sep;20(6):538-50. doi: 10.1111/odi.12159. Epub 2013 Jul 15. Oral Dis. 2014. PMID: 23849049 Review.
Cited by
-
The Effect of Thiamine, Ascorbic Acid, and the Combination of Them on the Levels of Matrix Metalloproteinase-9 (MMP-9) and Tissue Inhibitor of Matrix Metalloproteinase-1 (TIMP-1) in Sepsis Patients.Infect Drug Resist. 2022 Sep 30;15:5741-5751. doi: 10.2147/IDR.S378523. eCollection 2022. Infect Drug Resist. 2022. PMID: 36204393 Free PMC article.
-
MMP-9 Concentration in Peritoneal Fluid Is a Valuable Biomarker Associated with Endotoxemia in Equine Colic.Mediators Inflamm. 2021 Jan 5;2021:9501478. doi: 10.1155/2021/9501478. eCollection 2021. Mediators Inflamm. 2021. PMID: 33488296 Free PMC article.
-
Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol. 2010 Jul;299(1):H25-35. doi: 10.1152/ajpheart.00620.2009. Epub 2010 Apr 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20382857 Free PMC article.
-
Tissue inhibitor of metalloproteinases-4. The road less traveled.Mol Cancer. 2008 Nov 21;7:85. doi: 10.1186/1476-4598-7-85. Mol Cancer. 2008. PMID: 19025595 Free PMC article. Review.
-
Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte.Br J Pharmacol. 2008 Feb;153(4):676-83. doi: 10.1038/sj.bjp.0707621. Epub 2007 Dec 10. Br J Pharmacol. 2008. PMID: 18071296 Free PMC article.
References
-
- Albert J, Radomski A, Soop A, Sollevi A, Frostell C, Radomski MW. Differential release of matrix metalloproteinase-9 and nitric oxide following infusion of endotoxin to human volunteers. Acta Anaesthesiol Scand. 2003;47:407–410. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good the bad, and the ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. - PubMed
-
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. - PubMed
-
- Cheung P-Y, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

