Multi-micronutrient Sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial
- PMID: 16881929
- PMCID: PMC6860742
- DOI: 10.1111/j.1740-8709.2006.00060.x
Multi-micronutrient Sprinkles including a low dose of iron provided as microencapsulated ferrous fumarate improves haematologic indices in anaemic children: a randomized clinical trial
Abstract
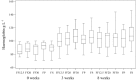
Home-fortification of complementary foods with micronutrients (including iron) as Sprinkles is a new strategy to control iron deficiency and anaemia in developing countries. However, the most effective dose and form of iron is not known. The purpose of this study was to compare the efficacy of various doses (12.5, 20 or 30 mg) and treatment methods (multi-micronutrient Sprinkles vs. ferrous sulphate drops) on haemoglobin (Hb) concentration after 8 weeks of treatment in anaemic children. In total, 133 anaemic Ghanaian children (Hb 70-99 g L(-1)) aged 6-18 months were randomly assigned to one of five daily interventions for 8 weeks. Out of the five interventions, four used Sprinkles, and one used iron drops. Of the four Sprinkles groups, three included 12.5, 20 or 30 mg of iron as ferrous fumarate, and one included 20 mg of iron as ferric pyrophosphate. The iron drops group included 12.5 mg of iron as liquid ferrous sulphate. Hb concentrations were measured at baseline, week 3 and week 8. The primary outcome measure was Hb concentration at 8 weeks after treatment. We compared differences in Hb and ferritin concentrations and prevalence of iron deficiency anaemia (Hb < 100 g L(-1) and soluble transferrin receptor concentrations >8.5 mg L(-1)) from baseline to 8 weeks within and between groups. Adherence and reporting of side effects (staining of the teeth, ease of use, diarrhoea and darkening of stools) were compared between groups. Mean change in Hb was 1.4 g L(-1) (SD = 1.8) (P = 0.0001). Change in Hb concentrations from baseline to 8 weeks was significant in all groups (P = 0.0001-0.0007), with no differences across groups. Geometric means of serum ferritin varied from 18.6 to 44.0 microg L(-1) at baseline. At week 8, these means were in the interval of 48.0-78.3 microg L(-1), with no group differences. Prevalence of iron deficiency anaemia decreased significantly from baseline to 8 weeks in all groups with the exception of the iron drops group, with no group differences. Adherence was lower in the drops group (64%) as compared with Sprinkles groups (84%). Greater staining of the teeth and less ease of use were reported in the drops group as compared with Sprinkles groups. A dose as low as 12.5 mg of iron as ferrous fumarate when provided as Sprinkles may be effective in anaemic children.
Figures

References
-
- Behrman J.R., Alderman H. & Hoddinott J. (2004) Copenhagen Consensus: Hunger and Malnutrition. Copenhagen Consesus: Copenhagen, Denmark.
-
- Brotanek J.M., Halterman J.S., Auinger P., Flores G. & Weitzman M. (2005) Iron deficiency, prolonged bottle‐feeding, and racial/ethnic disparities in young children. Archives of Pediatrics and Adolescent Medicine 159, 1038–1042. - PubMed
-
- Cohen A.R. & Seidl‐Friedman J. (1988) HemoCue system of hemoglobin measurement. Evaluation in anemic and nonanemic children. American Journal of Clinical Pathology 90, 302–305. - PubMed
-
- Davidsson L., Kastenmayer P., Szajewska H., Hurrell R.F. & Barclay D. (2000) Iron bioavailability in infants from an infant cereal fortified with ferric pyrophosphate or ferrous fumarate. American Journal of Clinical Nutrition 71, 1597–1602. - PubMed
-
- Fidler M.C., Walczyk T., Davidsson L., Zeder C., Sakaguchi N., Juneja L.R. et al. (2004) A micronised, dispersible ferric pyrophosphate with high relative bioavailability in man. British Journal of Nutrition 91, 107–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

