Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus
- PMID: 16882011
- PMCID: PMC2919221
- DOI: 10.1111/j.1460-9568.2006.04880.x
Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus
Abstract
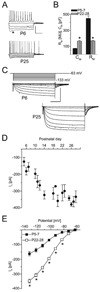
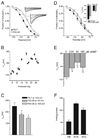
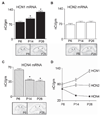
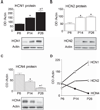
The hyperpolarization-activated current (I(h)) contributes to intrinsic properties and network responses of neurons. Its biophysical properties depend on the expression profiles of the underlying hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels and the presence of cyclic AMP (cAMP) that potently and differentially modulates I(h) conducted by HCN1, HCN2 and/or HCN4. Here, we studied the properties of I(h) in hippocampal CA1 pyramidal cells, the developmental evolution of the HCN-subunit isoforms that contribute to this current, and their interplay with age-dependent free cAMP concentrations, using electrophysiological, molecular and biochemical methods. I(h) amplitude increased progressively during the first four postnatal weeks, consistent with the observed overall increased expression of HCN channels. Activation kinetics of the current accelerated during this period, consonant with the quantitative reduction of mRNA and protein expression of the slow-kinetics HCN4 isoform and increased levels of HCN1. The sensitivity of I(h) to cAMP, and the contribution of the slow component to the overall I(h), decreased with age. These are likely a result of the developmentally regulated transition of the complement of HCN channel isoforms from cAMP sensitive to relatively cAMP insensitive. Thus, although hippocampal cAMP concentrations increased over twofold during the developmental period studied, the coordinated changes in expression of three HCN channel isoforms resulted in reduced effects of this signalling molecule on neuronal h currents.
Figures

Similar articles
-
Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus.Cereb Cortex. 2007 Mar;17(3):702-12. doi: 10.1093/cercor/bhk021. Epub 2006 Apr 28. Cereb Cortex. 2007. PMID: 16648453 Free PMC article.
-
Postnatal expression pattern of HCN channel isoforms in thalamic neurons: relationship to maturation of thalamocortical oscillations.J Neurosci. 2009 Jul 8;29(27):8847-57. doi: 10.1523/JNEUROSCI.0689-09.2009. J Neurosci. 2009. PMID: 19587292 Free PMC article.
-
Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide.J Gen Physiol. 2001 May;117(5):491-504. doi: 10.1085/jgp.117.5.491. J Gen Physiol. 2001. PMID: 11331358 Free PMC article.
-
The structure of the apo cAMP-binding domain of HCN4 - a stepping stone toward understanding the cAMP-dependent modulation of the hyperpolarization-activated cyclic-nucleotide-gated ion channels.FEBS J. 2018 Jun;285(12):2182-2192. doi: 10.1111/febs.14408. Epub 2018 Mar 14. FEBS J. 2018. PMID: 29444387 Review.
-
From funny current to HCN channels: 20 years of excitation.News Physiol Sci. 2002 Feb;17:32-7. doi: 10.1152/physiologyonline.2002.17.1.32. News Physiol Sci. 2002. PMID: 11821534 Review.
Cited by
-
Protein expression changes of HCN1 and HCN2 in hippocampal subregions of gerbils during the normal aging process.Iran J Basic Med Sci. 2019 Nov;22(11):1308-1313. doi: 10.22038/ijbms.2019.35760.8520. Iran J Basic Med Sci. 2019. PMID: 32128096 Free PMC article.
-
Loss of HCN2 in Dorsal Hippocampus of Young Adult Mice Induces Specific Apoptosis of the CA1 Pyramidal Neuron Layer.Int J Mol Sci. 2021 Jun 22;22(13):6699. doi: 10.3390/ijms22136699. Int J Mol Sci. 2021. PMID: 34206649 Free PMC article.
-
Ectopic HCN4 expression drives mTOR-dependent epilepsy in mice.Sci Transl Med. 2020 Nov 18;12(570):eabc1492. doi: 10.1126/scitranslmed.abc1492. Sci Transl Med. 2020. PMID: 33208499 Free PMC article.
-
Noradrenergic Suppression of Persistent Firing in Hippocampal CA1 Pyramidal Cells through cAMP-PKA Pathway.eNeuro. 2021 Mar 22;8(2):ENEURO.0440-20.2020. doi: 10.1523/ENEURO.0440-20.2020. Print 2021 Mar-Apr. eNeuro. 2021. PMID: 33637539 Free PMC article.
-
Cocaine sensitization increases I h current channel subunit 2 (HCN₂) protein expression in structures of the mesocorticolimbic system.J Mol Neurosci. 2013 May;50(1):234-45. doi: 10.1007/s12031-012-9920-4. Epub 2012 Dec 1. J Mol Neurosci. 2013. PMID: 23203153 Free PMC article.
References
-
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. - PubMed
-
- Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Differential and age-dependent expression of hyperpolarization- activated, cyclic nucleotide-gated cation channel isoforms 1–4 suggests evolving roles in the developing rat hippocampus. Neuroscience. 2001;106:689–698. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

