Rabs and their effectors: achieving specificity in membrane traffic
- PMID: 16882731
- PMCID: PMC1567661
- DOI: 10.1073/pnas.0601617103
Rabs and their effectors: achieving specificity in membrane traffic
Abstract
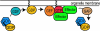
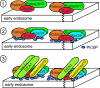
Rab proteins constitute the largest branch of the Ras GTPase superfamily. Rabs use the guanine nucleotide-dependent switch mechanism common to the superfamily to regulate each of the four major steps in membrane traffic: vesicle budding, vesicle delivery, vesicle tethering, and fusion of the vesicle membrane with that of the target compartment. These different tasks are carried out by a diverse collection of effector molecules that bind to specific Rabs in their GTP-bound state. Recent advances have not only greatly extended the number of known Rab effectors, but have also begun to define the mechanisms underlying their distinct functions. By binding to the guanine nucleotide exchange proteins that activate the Rabs certain effectors act to establish positive feedback loops that help to define and maintain tightly localized domains of activated Rab proteins, which then serve to recruit other effector molecules. Additionally, Rab cascades and Rab conversions appear to confer directionality to membrane traffic and couple each stage of traffic with the next along the pathway.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Geli M. I., Riezman H. J. Cell Sci. 1998;111:1031–1037. - PubMed
-
- Palade G. Science. 1975;189:347–358. - PubMed
-
- Rothman J. E. Nature. 1994;372:55–63. - PubMed
-
- Kartberg F., Elsner M., Froderberg L., Asp L., Nilsson T. Biochim. Biophys. Acta. 2005;1744:351–363. - PubMed
-
- Chavrier P., Goud B. Curr. Opin. Cell. Biol. 1999;11:466–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

