Biosynthesis of antitumoral and bactericidal sanguinarine
- PMID: 16883053
- PMCID: PMC1559923
- DOI: 10.1155/JBB/2006/63518
Biosynthesis of antitumoral and bactericidal sanguinarine
Abstract
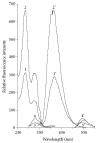
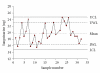
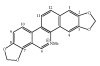
A simple, rapid, and reliable TLC method for the separation and determination of sanguinarine has been established. This intensively studied biologically active alkaloid has a wide range of potentially useful medicinal properties, such as antimicrobial, antiinflammatory, and antitumoral activities. Sanguinarine has also been incorporated into expectorant mixtures and has a strong bactericidal effect upon gram-positive bacteria, particularly Bacillus anthracis and staphylococci. These medicinal properties are due to the interaction of sanguinarine with DNA. A fibre-optic-based fluorescence instrument for in situ scanning was used for quantitative measurements. The sanguinarine was determined over the range 5-40 ng and a detection limit of 1.60 ng. The method was applied to the quantification of sanguinarine in tissue culture extracts of Chelidonium majus L.
Figures

References
-
- Fraga BM, García VP, González AG, Hernández MG, Hanson JR, Hitchcock PB. The dimerization of the chromene, precocene II. Journal of the Chemical Society. Perkin Transactions 1. 1983:2687–2693.
-
- Fraga BM, García VP. One-step preparation of the 3, 3′-dimer of precocene II. The Journal of Organic Chemistry. 1987;52(22):5032–5034.
-
- Fraga BM, Cabrera I, García VP, Guillermo R, Perales A. The acylation-dimerization of precocene II. Tetrahedron. 1997;53(47):16177–16184.
-
- Fraga BM, Cabrera I, García VP. On the facila dimerizatlon of precocene II. Heterocycles. 1999;51(11):2747–2752.
-
- Facchini PJ. Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:29–66. - PubMed
LinkOut - more resources
Full Text Sources

