Cloning and expression of human membrane-bound and soluble engineered T cell receptors for immunotherapy
- PMID: 16883054
- PMCID: PMC1510948
- DOI: 10.1155/JBB/2006/68091
Cloning and expression of human membrane-bound and soluble engineered T cell receptors for immunotherapy
Abstract
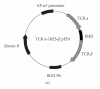
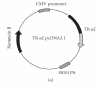
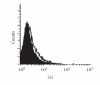
We report here the design and construction of several gene vectors for expression in mammalian cells of membrane-bound and soluble human T cell receptors (TR). We designed a vector (TR-ALPHA-IRES-TR-BETA pEF4) that encodes high-level expression of the full-length TR on the surface of T cells. Furthermore, we engineered TR that does not require the presence of endogenous CD3 molecules for surface expression and thus expression is not limited to T cells. We also constructed a vector encoding a single-chain TR (scTR) as a fusion protein of V-ALPHA-V-BETA-C-BETA with CD3Z. Since it is encoded and expressed as a single molecule, this scTR is well suited for gene therapy. Lastly, we successfully used a mammalian expression vector for generation of soluble human TR. The approaches we used here for manipulation of a human tumor-specific TR can be useful for other investigators interested in TR-based immunotherapy.
Figures












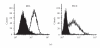
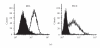
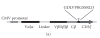
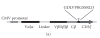
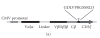







Similar articles
-
Suspension packaging cell lines for the simplified generation of T-cell receptor encoding retrovirus vector particles.Gene Ther. 2007 Apr;14(7):595-603. doi: 10.1038/sj.gt.3302906. Epub 2007 Jan 18. Gene Ther. 2007. PMID: 17235289
-
T cell receptor-alpha beta lacking the beta-chain V domain can be expressed at the cell surface but prohibits T cell maturation.J Immunol. 1992 Jun 15;148(12):3714-22. J Immunol. 1992. PMID: 1351085
-
[Construction and identification of recombinant adenovirus vector containing thioredoxin reductase gene].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003 Sep;19(5):440-2. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2003. PMID: 15169650 Chinese.
-
A recombinant single-chain human class II MHC molecule (HLA-DR1) as a covalently linked heterotrimer of alpha chain, beta chain, and antigenic peptide, with immunogenicity in vitro and reduced affinity for bacterial superantigens.Eur J Immunol. 1997 Aug;27(8):1933-41. doi: 10.1002/eji.1830270817. Eur J Immunol. 1997. PMID: 9295029
-
Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR.Cancer Res. 2009 Dec 1;69(23):9003-11. doi: 10.1158/0008-5472.CAN-09-1450. Epub 2009 Nov 10. Cancer Res. 2009. PMID: 19903853
Cited by
-
Lentiviral vectors encoding human MUC1-specific, MHC-unrestricted single-chain TCR and a fusion suicide gene: potential for universal and safe cancer immunotherapy.Cancer Immunol Immunother. 2009 Jun;58(6):977-87. doi: 10.1007/s00262-008-0624-0. Epub 2008 Nov 21. Cancer Immunol Immunother. 2009. PMID: 19023569 Free PMC article.
References
-
- Rubinstein MP, Kadima AN, Salem ML, et al. Transfer of TCR genes into mature T cells is accompanied by the maintenance of parental T cell avidity. The Journal of Immunology. 2003;170(3):1209–1217. - PubMed
-
- Morgan RA, Dudley ME, Yu YY, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. The Journal of Immunology. 2003;171(6):3287–3295. - PMC - PubMed
-
- Aarnoudse CA, Krüse M, Konopitzky R, Brouwenstijn N, Schrier PI. TCR reconstitution in Jurkat reporter cells facilitates the identification of novel tumor antigens by cDNA expression cloning. International Journal of Cancer. 2002;99(1):7–13. - PubMed
-
- Derby MA, Wang J, Margulies DH, Berzofsky JA. Two intermediate-avidity cytotoxic T lymphocyte clones with a disparity between functional avidity and MHC tetramer staining. International Immunology. 2001;13(6):817–824. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

