LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in daqingye and banlangen
- PMID: 16884885
- PMCID: PMC7126482
- DOI: 10.1016/j.jpba.2006.06.029
LC-APCI-MS method for detection and analysis of tryptanthrin, indigo, and indirubin in daqingye and banlangen
Erratum in
- J Pharm Biomed Anal. 2007 Jul 27;44(3):829
Abstract
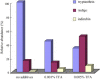
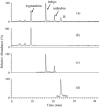
A rapid, selective, and sensitive LC-APCI-MS method is developed in this study for detecting and analyzing tryptanthrin, indigo, and indirubin in daqingye and banlangen, which are, respectively, the leaves and roots of Isatis indigotica and Strobilanthes cusia in traditional Chinese medicine. The detection of the three active components is linear in concentrations ranging from 100 to 1500 ng/mL, the squared correlation coefficient is higher than 0.996, the precision as measured by the relative standard deviation is no larger than 9.5%, and the recovery is greater than 86.6%. The analysis of the 21 banlangen samples led to considerably different conclusions on the contents of tryptanthrin, indigo, and indirubin in fresh leaves versus those in dried leaves. These results should shed some light on future plant selection and breeding. Compared with the traditional TLC and HPLC-UV methods, the new LC-APCI-MS approach has proven to be an optimal tool for detecting and analyzing the three marker compounds in the Chinese herbal medicines of daqingye and banlangen.
Figures


References
-
- Pan Z.A., Sun X.F., editors. Chinese Pharmacopoeia. first ed. Council of China; Beijing: 2005. pp. 142–143.
-
- Ho Y.L., Chang Y.S. Phytomedicine. 2002;9:419–424. - PubMed
-
- Wu X., Liu Y., Sheng W., Sun J., Qin G. Planta Med. 1997;63:55–57. - PubMed
-
- Liu Y.H., Qin G.W., Ding S.P., Wu X.Y. Chin. Tradit. Herbal Drugs. 2001:1057–1060.
-
- Liu Y.H., Qin G.W., Ding S.P., Wu X.Y. Chin. Tradit. Herbal Drugs. 2002;33:97–99.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

