Adenosine and sleep homeostasis in the Basal forebrain
- PMID: 16885223
- PMCID: PMC6673779
- DOI: 10.1523/JNEUROSCI.2181-06.2006
Adenosine and sleep homeostasis in the Basal forebrain
Abstract
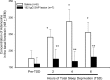
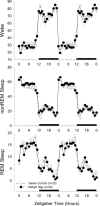
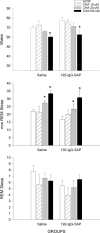
It is currently hypothesized that the drive to sleep is determined by the activity of the basal forebrain (BF) cholinergic neurons, which release adenosine (AD), perhaps because of increased metabolic activity associated with the neuronal discharge during waking, and the accumulating AD begins to inhibit these neurons so that sleep-active neurons can become active. This hypothesis grew from the observation that AD induces sleep and AD levels increase with wake in the basal forebrain, but surprisingly it still remains untested. Here we directly test whether the basal forebrain cholinergic neurons are central to the AD regulation of sleep drive by administering 192-IgG-saporin to lesion the BF cholinergic neurons and then measuring AD levels in the BF. In rats with 95% lesion of the BF cholinergic neurons, AD levels in the BF did not increase with 6 h of prolonged waking. However, the lesioned rats had intact sleep drive after 6 and 12 h of prolonged waking, indicating that the AD accumulation in the BF is not necessary for sleep drive. Next we determined that, in the absence of the BF cholinergic neurons, the selective adenosine A1 receptor agonist N6-cyclohexyladenosine, administered to the BF, continued to be effective in inducing sleep, indicating that the BF cholinergic neurons are not essential to sleep induction. Thus, neither the activity of the BF cholinergic neurons nor the accumulation of AD in the BF during wake is necessary for sleep drive.
Figures




References
-
- Alanko L, Heiskanen S, Stenberg D, Porkka-Heiskanen T (2003). Adenosine kinase and 5′-nucleotidase activity after prolonged wakefulness in the cortex and the basal forebrain of rat. Neurochem Int 42:449–454. - PubMed
-
- Bassant MH, Apartis E, Jazat-Poindessous FR, Wiley RG, Lamour YA (1995). Selective immunolesion of the basal forebrain cholinergic neurons: effects on hippocampal activity during sleep and wakefulness in the rat. Neurodegeneration 4:61–70. - PubMed
-
- Book AA, Wiley RG, Schweitzer JB (1994). 192 IgG-saporin. I. Specific lethality for cholinergic neurons in the basal forebrain of the rat. J Neuropathol Exp Neurol 53:95–102. - PubMed
-
- Borbely AA, Tobler I, Hanagasioglu M (1984). Effect of sleep deprivation on sleep and EEG power spectra in the rat. Behav Brain Res 14:171–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
