Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons
- PMID: 16885227
- PMCID: PMC6673772
- DOI: 10.1523/JNEUROSCI.1183-06.2006
Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons
Abstract
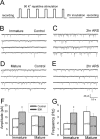
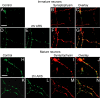
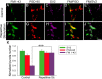
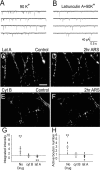
Developing neurons have greater capacity in experience-dependent plasticity than adult neurons but the molecular mechanism is not well understood. Here we report a developmentally regulated long-term synaptic plasticity through actin-dependent activation of presynaptic silent synapses in cultured hippocampal neurons. Live FM 1-43 imaging and retrospective immunocytochemistry revealed that many presynaptic boutons in immature neurons are functionally silent at resting conditions, but can be converted into active ones after repetitive neuronal stimulation. The activation of presynaptic silent synapses is dependent on L-type calcium channels and protein kinase A (PKA)/PKC signaling pathways. Moreover, blocking actin polymerization with latrunculin A and cytochalasin B abolishes long-term increase of presynaptic functional boutons induced by repetitive stimulation, whereas actin polymerizer jasplakinolide increases the number of active boutons in immature neurons. In mature neurons, however, presynaptic boutons are mostly functional and repetitive stimulation did not induce additional enhancement. Quantitative immunostaining with phalloidin revealed a significant increase in axonal F-actin level after repetitive stimulation in immature but not mature neurons. These results suggest that actin-dependent activation of presynaptic silent synapses contributes significantly to the long-term synaptic plasticity during neuronal development.
Figures


Similar articles
-
Synaptic vesicles in rat hippocampal boutons recycle to different pools in a use-dependent fashion.J Physiol. 2006 May 1;572(Pt 3):707-20. doi: 10.1113/jphysiol.2005.100842. J Physiol. 2006. PMID: 16439431 Free PMC article.
-
Kinase activity is not required for alphaCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses.Nat Neurosci. 2007 Sep;10(9):1125-7. doi: 10.1038/nn1946. Epub 2007 Jul 29. Nat Neurosci. 2007. PMID: 17660813 Free PMC article.
-
Presynaptic CaMKII is necessary for synaptic plasticity in cultured hippocampal neurons.Neuron. 2004 Apr 8;42(1):129-41. doi: 10.1016/s0896-6273(04)00143-6. Neuron. 2004. PMID: 15066270
-
Presynaptically silent synapses: dormancy and awakening of presynaptic vesicle release.Neuroscientist. 2012 Jun;18(3):216-23. doi: 10.1177/1073858411418525. Epub 2011 Sep 9. Neuroscientist. 2012. PMID: 21908849 Free PMC article. Review.
-
Presynaptic facilitation of synaptic transmission in the hippocampus.Pharmacol Ther. 1998 Mar;77(3):203-23. doi: 10.1016/s0163-7258(97)00162-9. Pharmacol Ther. 1998. PMID: 9576628 Review.
Cited by
-
Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures.J Neurosci. 2007 Oct 3;27(40):10860-9. doi: 10.1523/JNEUROSCI.2744-07.2007. J Neurosci. 2007. PMID: 17913919 Free PMC article.
-
BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses.Exp Brain Res. 2009 Dec;199(3-4):203-34. doi: 10.1007/s00221-009-1994-z. Epub 2009 Sep 24. Exp Brain Res. 2009. PMID: 19777221 Review.
-
Amyloid fibrils induce dysfunction of hippocampal glutamatergic silent synapses.Hippocampus. 2018 Aug;28(8):549-556. doi: 10.1002/hipo.22955. Epub 2018 May 7. Hippocampus. 2018. PMID: 29704282 Free PMC article.
-
Rapid sequential clustering of NMDARs, CaMKII, and AMPARs upon activation of NMDARs at developing synapses.Front Synaptic Neurosci. 2024 Apr 10;16:1291262. doi: 10.3389/fnsyn.2024.1291262. eCollection 2024. Front Synaptic Neurosci. 2024. PMID: 38660466 Free PMC article.
-
Histone deacetylases 1 and 2 form a developmental switch that controls excitatory synapse maturation and function.J Neurosci. 2009 Jun 24;29(25):8288-97. doi: 10.1523/JNEUROSCI.0097-09.2009. J Neurosci. 2009. PMID: 19553468 Free PMC article.
References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R (1997). Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615–626. - PubMed
-
- Ackermann M, Matus A (2003). Activity-induced targeting of profilin and stabilization of dendritic spine morphology. Nat Neurosci 6:1194–1200. - PubMed
-
- Ahmari SE, Buchanan J, Smith SJ (2000). Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci 3:445–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
