Unlocking Streptomyces spp. for use as sustainable industrial production platforms by morphological engineering
- PMID: 16885277
- PMCID: PMC1538695
- DOI: 10.1128/AEM.00808-06
Unlocking Streptomyces spp. for use as sustainable industrial production platforms by morphological engineering
Erratum in
- Appl Environ Microbiol. 2006 Oct;72(10):6863
Abstract
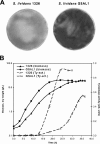
Filamentous actinomycetes are commercially widely used as producers of natural products (in particular antibiotics) and of industrial enzymes. However, the mycelial lifestyle of actinomycetes, resulting in highly viscous broths and unfavorable pellet formation, has been a major bottleneck in their commercialization. Here we describe the successful morphological engineering of industrially important streptomycetes through controlled expression of the morphogene ssgA. This led to improved growth of many industrial and reference streptomycetes, with fragmentation of the mycelial clumps resulting in significantly enhanced growth rates in batch fermentations of Streptomyces coelicolor and Streptomyces lividans. Product formation was also stimulated, with a twofold increase in yield of enzyme production by S. lividans. We anticipate that the use of the presented methodology will make actinomycetes significantly more attractive as industrial and sustainable production hosts.
Figures



Similar articles
-
Morphogenesis of Streptomyces in submerged cultures.Adv Appl Microbiol. 2014;89:1-45. doi: 10.1016/B978-0-12-800259-9.00001-9. Adv Appl Microbiol. 2014. PMID: 25131399 Review.
-
Actinorhodin production by Streptomyces coelicolor and growth of Streptomyces lividans are improved by the expression of a bacterial hemoglobin.Biotechnology (N Y). 1991 May;9(5):473-6. doi: 10.1038/nbt0591-473. Biotechnology (N Y). 1991. PMID: 1367312
-
Enhancement and selective production of oligomycin through inactivation of avermectin's starter unit in Streptomyces avermitilis.Biotechnol Lett. 2006 Jun;28(12):911-6. doi: 10.1007/s10529-006-9012-z. Epub 2006 May 24. Biotechnol Lett. 2006. PMID: 16786277
-
Enhanced production of heterologous macrolide aglycones by fed-batch cultivation of Streptomyces coelicolor.J Ind Microbiol Biotechnol. 2002 May;28(5):297-301. doi: 10.1038/sj/jim/7000246. J Ind Microbiol Biotechnol. 2002. PMID: 11986935
-
Biotechnological doxorubicin production: pathway and regulation engineering of strains for enhanced production.Appl Microbiol Biotechnol. 2010 Jul;87(4):1187-94. doi: 10.1007/s00253-010-2675-3. Epub 2010 May 28. Appl Microbiol Biotechnol. 2010. PMID: 20508927 Review.
Cited by
-
Peripheral and Central Glutamate Dyshomeostasis in Neurodegenerative Disorders.Curr Neuropharmacol. 2021;19(7):1069-1089. doi: 10.2174/1570159X18666201015161919. Curr Neuropharmacol. 2021. PMID: 33059576 Free PMC article.
-
Reviewing a plethora of oxidative-type reactions catalyzed by whole cells of Streptomyces species.RSC Adv. 2022 Mar 1;12(12):6974-7001. doi: 10.1039/d1ra08816e. eCollection 2022 Mar 1. RSC Adv. 2022. PMID: 35424663 Free PMC article. Review.
-
Positive control of cell division: FtsZ is recruited by SsgB during sporulation of Streptomyces.Genes Dev. 2011 Jan 1;25(1):89-99. doi: 10.1101/gad.600211. Genes Dev. 2011. PMID: 21205868 Free PMC article.
-
Feast or famine: the global regulator DasR links nutrient stress to antibiotic production by Streptomyces.EMBO Rep. 2008 Jul;9(7):670-5. doi: 10.1038/embor.2008.83. Epub 2008 May 30. EMBO Rep. 2008. PMID: 18511939 Free PMC article.
-
Characterization of Amycolatopsis 75iv2 dye-decolorizing peroxidase on O-glycosides.Appl Environ Microbiol. 2024 May 21;90(5):e0020524. doi: 10.1128/aem.00205-24. Epub 2024 Apr 16. Appl Environ Microbiol. 2024. PMID: 38625022 Free PMC article.
References
-
- Bai, Z., L. M. Harvey, and B. McNeil. 2003. Oxidative stress in submerged cultures of fungi. Crit. Rev. Biotechnol. 23:267-302. - PubMed
-
- Bennett, J. W. 1998. Mycotechnology: the role of fungi in biotechnology. J. Biotechnol. 66:101-107. - PubMed
-
- Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. - PubMed
-
- Bibb, M. 1996. 1995 Colworth Prize lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335-1344. - PubMed
-
- Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

