Genetic diversity of Borrelia burgdorferi sensu stricto in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in southern Maryland
- PMID: 16885284
- PMCID: PMC1538722
- DOI: 10.1128/AEM.00014-06
Genetic diversity of Borrelia burgdorferi sensu stricto in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in southern Maryland
Abstract
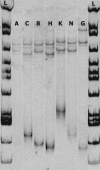
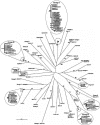
In the north central and northeastern United States, Borrelia burgdorferi sensu stricto, the etiologic agent of Lyme disease (LD), is maintained in an enzootic cycle between the vector, Ixodes scapularis, and the primary reservoir host, Peromyscus leucopus. Genetic diversity of the pathogen based on sequencing of two plasmid-located genes, those for outer surface protein A (ospA) and outer surface protein C (ospC), has been examined in both tick and human specimens at local, regional, and worldwide population scales. Additionally, previous studies have only been conducted with tick or human specimens at the local population level in areas with high LD transmission rates. This study examined the genetic diversity of circulating borreliae in the reservoir population from a large region of the western coastal plains of southern Maryland, where moderate numbers of human LD cases are reported. Six ospA mobility classes, including two that were not previously described, and eight ospC groups were found among the P. leucopus samples. Twenty-five percent of all specimens were infected with more than one ospA or ospC variant. The frequency distribution of variants was homogeneous, both locally and spatially. The spirochete diversity found in Maryland was not as high as that observed among northern tick populations, yet similar genotypes were observed in both populations. These results also show that mice are important for maintaining Borrelia variants, even rare variants, and that reservoir populations should therefore be considered when assessing the diversity of B. burgdorferi.
Figures

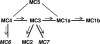
References
-
- Alghaferi, M. Y., J. M. Anderson, J. Park, P. G. Auwaerter, J. N. Aucott, D. E. Norris, and J. S. Dumler. 2005. Borrelia burgdorferi ospC heterogeneity among human and murine isolates from a defined region of northern Maryland and southern Pennsylvania: lack of correlation with invasive and noninvasive genotypes. J. Clin. Microbiol. 43:1879-1884. - PMC - PubMed
-
- Barbour, A. G., and C. F. Garon. 1988. The genes encoding major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann. N. Y. Acad. Sci. 539:144-153. - PubMed
-
- Bunikis, J., U. Garpmo, J. Tsao, J. Berglund, D. Fish, and A. G. Barbour. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741-1755. - PubMed
-
- Bunikis, J., J. Tasao, C. Luke, M. Luna, D. Fish, and A. G. Barbour. 2004. Borrelia burgdorferi infection in natural populations of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. J. Infect. Dis. 189:1515-1523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

