The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines
- PMID: 16885441
- PMCID: PMC1540089
- DOI: 10.1128/JB.00495-06
The Bordetella bfe system: growth and transcriptional response to siderophores, catechols, and neuroendocrine catecholamines
Abstract
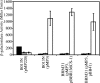
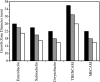
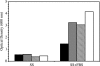
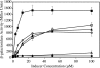
Ferric enterobactin utilization by Bordetella bronchiseptica and Bordetella pertussis requires the BfeA outer membrane receptor. Under iron-depleted growth conditions, transcription of bfeA is activated by the BfeR regulator by a mechanism requiring the siderophore enterobactin. In this study, enterobactin-inducible bfeA transcription was shown to be TonB independent. To determine whether other siderophores or nonsiderophore catechols could be utilized by the Bfe system, various compounds were tested for the abilities to promote the growth of iron-starved B. bronchiseptica and induce bfeA transcription. The BfeA receptor transported ferric salmochelin, corynebactin, and the synthetic siderophores TRENCAM and MECAM. Salmochelin and MECAM induced bfeA transcription in iron-starved Bordetella cells, but induction by corynebactin and TRENCAM was minimal. The neuroendocrine catecholamines epinephrine, norepinephrine, and dopamine exhibited a remarkable capacity to induce transcription of bfeA. Norepinephrine treatment of B. bronchiseptica resulted in BfeR-dependent bfeA transcription, elevated BfeA receptor production, and growth stimulation. Pyrocatechol, carbidopa, and isoproterenol were similarly strong inducers of bfeA transcription, whereas tyramine and 3,4-dihydroxymandelic acid demonstrated low inducing activity. The results indicate that the inducer structure requires a catechol group for function and that the ability to induce bfeA transcription does not necessarily correlate with the ability to stimulate bacterial growth. The expanded range of catechol siderophores transported by the BfeA receptor demonstrates the potential versatility of the Bordetella Bfe iron retrieval system. The finding that catecholamine neurotransmitters activate bfeA transcription and promote growth suggests that Bordetella cells can perceive and may benefit from neuroendocrine catecholamines on the respiratory epithelium.
Figures



Similar articles
-
The BfeR regulator mediates enterobactin-inducible expression of Bordetella enterobactin utilization genes.J Bacteriol. 2004 Nov;186(21):7302-11. doi: 10.1128/JB.186.21.7302-7311.2004. J Bacteriol. 2004. PMID: 15489442 Free PMC article.
-
Involvement of multiple distinct Bordetella receptor proteins in the utilization of iron liberated from transferrin by host catecholamine stress hormones.Mol Microbiol. 2012 May;84(3):446-62. doi: 10.1111/j.1365-2958.2012.08032.x. Epub 2012 Mar 27. Mol Microbiol. 2012. PMID: 22458330 Free PMC article.
-
An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors.Microbiology (Reading). 1997 Jan;143 ( Pt 1):135-145. doi: 10.1099/00221287-143-1-135. Microbiology (Reading). 1997. PMID: 9025287
-
Bordetella iron transport and virulence.Biometals. 2007 Jun;20(3-4):303-22. doi: 10.1007/s10534-006-9031-1. Epub 2007 Feb 13. Biometals. 2007. PMID: 17295050 Review.
-
Temporal signaling and differential expression of Bordetella iron transport systems: the role of ferrimones and positive regulators.Biometals. 2009 Feb;22(1):33-41. doi: 10.1007/s10534-008-9189-9. Epub 2009 Jan 7. Biometals. 2009. PMID: 19130264 Free PMC article. Review.
Cited by
-
Iron and pH-responsive FtrABCD ferrous iron utilization system of Bordetella species.Mol Microbiol. 2012 Nov;86(3):580-93. doi: 10.1111/mmi.12003. Epub 2012 Sep 11. Mol Microbiol. 2012. PMID: 22924881 Free PMC article.
-
Global effects of catecholamines on Actinobacillus pleuropneumoniae gene expression.PLoS One. 2012;7(2):e31121. doi: 10.1371/journal.pone.0031121. Epub 2012 Feb 8. PLoS One. 2012. PMID: 22347439 Free PMC article.
-
Blockade of catecholamine-induced growth by adrenergic and dopaminergic receptor antagonists in Escherichia coli O157:H7, Salmonella enterica and Yersinia enterocolitica.BMC Microbiol. 2007 Jan 30;7:8. doi: 10.1186/1471-2180-7-8. BMC Microbiol. 2007. PMID: 17263883 Free PMC article.
-
Communication between Bacteria and Their Hosts.Scientifica (Cairo). 2013;2013:361073. doi: 10.1155/2013/361073. Epub 2013 Dec 8. Scientifica (Cairo). 2013. PMID: 24381789 Free PMC article. Review.
-
Microbial telesensing: probing the environment for friends, foes, and food.Cell Host Microbe. 2009 Aug 20;6(2):115-24. doi: 10.1016/j.chom.2009.07.004. Cell Host Microbe. 2009. PMID: 19683678 Free PMC article. Review.
References
-
- Arima, K., A. Kakinuma, and G. Tamura. 1968. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 31:488-494. - PubMed
-
- Armstrong, S. K., C. L. Francis, and M. A. McIntosh. 1990. Molecular analysis of the Escherichia coli ferric enterobactin receptor FepA. J. Biol. Chem. 265:14536-14543. - PubMed
-
- Arnow, L. E. 1937. Colorimetric determination of the components of 3,4-dihydroxyphenylalanine-tyrosine mixtures. J. Biol. Chem. 118:531-537.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

