Cyclic AMP-dependent osmoregulation of crp gene expression in Escherichia coli
- PMID: 16885462
- PMCID: PMC1540082
- DOI: 10.1128/JB.00235-06
Cyclic AMP-dependent osmoregulation of crp gene expression in Escherichia coli
Abstract
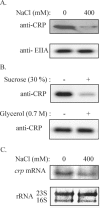
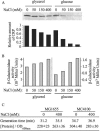
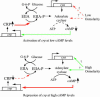
We have found that the cyclic AMP (cAMP) receptor protein (CRP)-cAMP regulatory complex in Escherichia coli is subject to osmoregulation at the level of crp gene expression. This osmoregulation was lost in a cya mutant strain but could be restored by external addition of cAMP, suggesting that the intracellular level of cAMP is a key factor in the osmoregulation of CRP. The ability of the cell to maintain optimal CRP activity was essential for the growth and survival of the bacteria under low-osmolarity conditions as shown by studies with different crp mutant alleles. A suppressor mutant with a novel amino acid substitution (L124R) in CRP showed restored growth at low osmolarity. CRP(L124R) was not activated by cAMP and was shown to be dominant negative over the wild type. Our findings suggest that the fine-tuning of the CRP activity may be critical for bacterial viability and adaptability to changing osmotic conditions.
Figures




Similar articles
-
Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP*cAMP complex.Nucleic Acids Res. 2005 Nov 27;33(21):6712-22. doi: 10.1093/nar/gki978. Print 2005. Nucleic Acids Res. 2005. PMID: 16314304 Free PMC article.
-
Cyclic AMP (cAMP) and cAMP receptor protein influence both synthesis and uptake of extracellular autoinducer 2 in Escherichia coli.J Bacteriol. 2005 Mar;187(6):2066-76. doi: 10.1128/JB.187.6.2066-2076.2005. J Bacteriol. 2005. PMID: 15743955 Free PMC article.
-
Positive Effect of Carbon Sources on Natural Transformation in Escherichia coli: Role of Low-Level Cyclic AMP (cAMP)-cAMP Receptor Protein in the Derepression of rpoS.J Bacteriol. 2015 Oct;197(20):3317-28. doi: 10.1128/JB.00291-15. Epub 2015 Aug 10. J Bacteriol. 2015. PMID: 26260461 Free PMC article.
-
Syn, anti, and finally both conformations of cyclic AMP are involved in the CRP-dependent transcription initiation mechanism in E. coli lac operon.Cell Biochem Funct. 2008 Jun;26(4):399-405. doi: 10.1002/cbf.1462. Cell Biochem Funct. 2008. PMID: 18338329 Review.
-
cAMP Activation of the cAMP Receptor Protein, a Model Bacterial Transcription Factor.J Microbiol. 2023 Mar;61(3):277-287. doi: 10.1007/s12275-023-00028-6. Epub 2023 Mar 9. J Microbiol. 2023. PMID: 36892777 Review.
Cited by
-
Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP.PLoS Pathog. 2009 Feb;5(2):e1000303. doi: 10.1371/journal.ppat.1000303. Epub 2009 Feb 20. PLoS Pathog. 2009. PMID: 19229313 Free PMC article.
-
Posttranscriptional regulation of the Yersinia pestis cyclic AMP receptor protein Crp and impact on virulence.mBio. 2014 Feb 11;5(1):e01038-13. doi: 10.1128/mBio.01038-13. mBio. 2014. PMID: 24520064 Free PMC article.
-
What Flips the Switch? Signals and Stress Regulating Extraintestinal Pathogenic Escherichia coli Type 1 Fimbriae (Pili).Microorganisms. 2021 Dec 21;10(1):5. doi: 10.3390/microorganisms10010005. Microorganisms. 2021. PMID: 35056454 Free PMC article. Review.
-
Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts.Nat Commun. 2023 Nov 6;14(1):7135. doi: 10.1038/s41467-023-42788-0. Nat Commun. 2023. PMID: 37932270 Free PMC article.
-
Metabolic shift of Escherichia coli under salt stress in the presence of glycine betaine.Appl Environ Microbiol. 2014 Aug;80(15):4745-56. doi: 10.1128/AEM.00599-14. Appl Environ Microbiol. 2014. PMID: 24858086 Free PMC article.
References
-
- Aiba, H. 1983. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell 32:141-149. - PubMed
-
- Aiba, H. 1985. Transcription of the Escherichia coli adenylate cyclase gene is negatively regulated by cAMP-cAMP receptor protein. J. Biol. Chem. 260:3063-3070. - PubMed
-
- Baga, M., M. Goransson, S. Normark, and B. E. Uhlin. 1988. Processed mRNA with differential stability in the regulation of E. coli pilin gene expression. Cell 52:197-206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

