Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation
- PMID: 16886058
- PMCID: PMC1525004
- DOI: 10.1172/JCI27179
Pathogenic role for skin macrophages in a mouse model of keratinocyte-induced psoriasis-like skin inflammation
Abstract
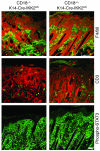
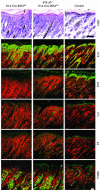
Psoriasis is a common skin disease, the pathogenesis of which has not yet been resolved. In mice, epidermis-specific deletion of inhibitor of NF-kappaB (IkappaB) kinase 2 (IKK2) results in a skin phenotype that mimics human psoriasis in several aspects. Like psoriasis, this skin disease shows pronounced improvement when mice are treated with a TNF-neutralizing agent. We have found previously that this phenotype does not depend on the presence of alphabeta T lymphocytes. In order to evaluate contributions of other immune cell populations to the skin disease, we selectively eliminated macrophages and granulocytes from the skin of mice with epidermis-specific deletion of IKK2 (K14-Cre-IKK2fl/fl mice). Elimination of skin macrophages by subcutaneous injection of clodronate liposomes was accompanied by inhibition of granulocyte migration into the skin and resulted in a dramatic attenuation of psoriasis-like skin changes. The hyperproliferative, inflammatory skin disease in K14-Cre-IKK2fl/fl mice was a direct consequence of the presence of macrophages in the skin, as targeted deletion of CD18, which prevented accumulation of granulocytes but not macrophages, did not lead to major changes in the phenotype. Targeted deletion of the receptor for IFN-gamma revealed that the pathogenesis of the skin disease does not depend on classical IFN-gamma-mediated macrophage activation. Our results demonstrate that in mice epidermal keratinocytes can initiate a hyperproliferative, inflammatory, IFN-gamma-independent, psoriasis-like skin disease whose development requires essential contributions from skin macrophages but not from granulocytes or alphabeta T lymphocytes.
Figures
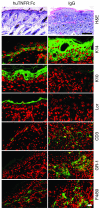
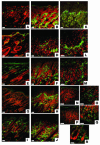
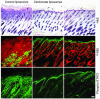

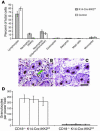

Comment in
-
Misbehaving macrophages in the pathogenesis of psoriasis.J Clin Invest. 2006 Aug;116(8):2084-7. doi: 10.1172/JCI29441. J Clin Invest. 2006. PMID: 16886055 Free PMC article.
References
-
- Lever, W.F., and Schaumburg-Lever, G. 1983. Histopathology of the skin. J.B. Lippincott Company. Philadelphia, Pennsylvania, USA. 848 pp.
-
- Schön M., et al. Critical role of neutrophils for the generation of psoriais skin lesions in flaky skin mice. J. Invest. Dermatol. 2000;114:976–983. - PubMed
-
- Schön M.P., Detmar M., Parker C.M. Murine psoriasis-like disorder induced by naive CD4+ T-cells. Nat. Med. 1997;3:183–188. - PubMed
-
- Blessing M., Schirmacher P., Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J. Cell Biol. 1996;135:227–239. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

