Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation
- PMID: 16886059
- PMCID: PMC1523400
- DOI: 10.1172/JCI27180
Activated macrophages are essential in a murine model for T cell-mediated chronic psoriasiform skin inflammation
Abstract
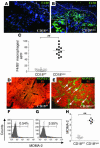
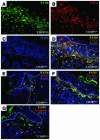
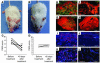
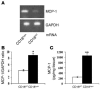
The CD18 hypomorphic (CD18hypo) PL/J mouse model clinically resembling human psoriasis is characterized by reduced expression of the common chain of beta2 integrins (CD11/CD18) to only 2-16% of WT levels. Previously we found that this chronic psoriasiform skin inflammation also depends on the presence of CD4+ T cells. Herein we investigated the role of macrophages in this CD18hypo mouse model. Activated macrophages were significantly increased in lesional skin as well as in inflamed skin draining lymph nodes (DLNs) of affected CD18hypo mice and were identified as being an important source of TNF-alpha in vivo. Both depletion of macrophages and neutralization of TNF-alpha resulted in a significant alleviation of psoriasiform skin inflammation. As monocyte chemotactic protein 1 was enhanced in lesional skin of affected CD18hypo mice, we intradermally injected recombinant murine monocyte chemotactic protein-1 (rJE/MCP-1) alone or in combination with rTNF-alpha into the skin of healthy CD18hypo mice. Only simultaneous injection of rJE/MCP-1 and rTNF-alpha, but neither substance alone, resulted in the induction of psoriasiform skin inflammation around the injection sites with recruitment and activation of macrophages. Collectively, our data suggest that maintenance of psoriasiform skin inflammation critically depends on efficient recruitment and activation of macrophages with sufficient release of TNF-alpha.
Figures

Comment in
-
Misbehaving macrophages in the pathogenesis of psoriasis.J Clin Invest. 2006 Aug;116(8):2084-7. doi: 10.1172/JCI29441. J Clin Invest. 2006. PMID: 16886055 Free PMC article.
References
-
- Nickoloff B.J. The immunologic and genetic basis of psoriasis. Arch. Dermatol. 1999;135:1104–1110. - PubMed
-
- Gillitzer R., et al. MCP-1 mRNA expression in basal keratinocytes of psoriatic lesions. J. Invest. Dermatol. 1993;101:127–131. - PubMed
-
- van den Oord J.J., de Wolf-Peeters C. Epithelium-lining macrophages in psoriasis. Br. J. Dermatol. 1994;130:589–594. - PubMed
-
- Djemadji-Oudjiel N., Goerdt S., Kodelja V., Schmuth M., Orfanos C.E. Immunohistochemical identification of type II alternatively activated dendritic macrophages (RM 3/1+3, MS-1+/–, 25F9-) in psoriatic dermis. Arch. Dermatol. Res. 1996;288:757–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

