Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance
- PMID: 16889645
- PMCID: PMC3233234
- DOI: 10.1111/j.1365-313X.2006.02837.x
Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance
Abstract
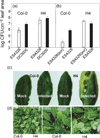
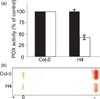
The oxidative burst is an early response to pathogen attack leading to the production of reactive oxygen species (ROS) including hydrogen peroxide. Two major mechanisms involving either NADPH oxidases or peroxidases that may exist singly or in combination in different plant species have been proposed for the generation of ROS. We identified an Arabidopsis thaliana azide-sensitive but diphenylene iodonium-insensitive apoplastic oxidative burst that generates H(2)O(2) in response to a Fusarium oxysporum cell-wall preparation. Transgenic Arabidopsis plants expressing an anti-sense cDNA encoding a type III peroxidase, French bean peroxidase type 1 (FBP1) exhibited an impaired oxidative burst and were more susceptible than wild-type plants to both fungal and bacterial pathogens. Transcriptional profiling and RT-PCR analysis showed that the anti-sense (FBP1) transgenic plants had reduced levels of specific peroxidase-encoding mRNAs, including mRNAs corresponding to Arabidopsis genes At3g49120 (AtPCb) and At3g49110 (AtPCa) that encode two class III peroxidases with a high degree of homology to FBP1. These data indicate that peroxidases play a significant role in generating H(2)O(2) during the Arabidopsis defense response and in conferring resistance to a wide range of pathogens.
Figures




References
-
- Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;93:773–784. - PubMed
-
- Apel K, Hirt H. reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004;55:373–399. - PubMed
-
- Bennett M, Mehta M, Grant M. Biophoton imaging; a non-destructive method for assaying R gene responses. Mol. Plant Microb. Interact. 2005;18:95–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

