Experimental evaluation of the FluChip diagnostic microarray for influenza virus surveillance
- PMID: 16891504
- PMCID: PMC1594652
- DOI: 10.1128/JCM.00134-06
Experimental evaluation of the FluChip diagnostic microarray for influenza virus surveillance
Abstract
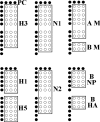
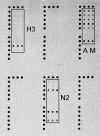
Global surveillance of influenza is critical for improvements in disease management and is especially important for early detection, rapid intervention, and a possible reduction of the impact of an influenza pandemic. Enhanced surveillance requires rapid, robust, and inexpensive analytical techniques capable of providing a detailed analysis of influenza virus strains. Low-density oligonucleotide microarrays with highly multiplexed "signatures" for influenza viruses offer many of the desired characteristics. However, the high mutability of the influenza virus represents a design challenge. In order for an influenza virus microarray to be of utility, it must provide information for a wide range of viral strains and lineages. The design and characterization of an influenza microarray, the FluChip-55 microarray, for the relatively rapid identification of influenza A virus subtypes H1N1, H3N2, and H5N1 are described here. In this work, a small set of sequences was carefully selected to exhibit broad coverage for the influenza A and B viruses currently circulating in the human population as well as the avian A/H5N1 virus that has become enzootic in poultry in Southeast Asia and that has recently spread to Europe. A complete assay involving extraction and amplification of the viral RNA was developed and tested. In a blind study of 72 influenza virus isolates, RNA from a wide range of influenza A and B viruses was amplified, hybridized, labeled with a fluorophore, and imaged. The entire analysis time was less than 12 h. The combined results for two assays provided the absolutely correct types and subtypes for an average of 72% of the isolates, the correct type and partially correct subtype information for 13% of the isolates, the correct type only for 10% of the isolates, false-negative signals for 4% of the isolates, and false-positive signals for 1% of the isolates. In the overwhelming majority of cases in which incomplete subtyping was observed, the failure was due to the nucleic acid amplification step rather than limitations in the microarray.
Figures


References
-
- Amano, Y., and Q. Cheng. 2005. Detection of influenza virus: traditional approaches and development of biosensors. Anal. Bioanal Chem. 381:156-164. - PubMed
-
- Anonymous. March. 31, 2006, posting date. Biological diagnostics manufacturing: request for information. U.S. Department of Health and Human Services Solicitation 2006-Q-08478. [Online.] http://www.fbo.gov/spg/HHS/CDCP/PGOA/2006%2DQ%2D08478/SynopsisR.html.
-
- Banks, J., E. Speidel, and D. J. Alexander. 1998. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch. Virol. 143:781-787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

