False-positive results of enzyme immunoassays for human immunodeficiency virus in patients with uncomplicated malaria
- PMID: 16891532
- PMCID: PMC1594619
- DOI: 10.1128/JCM.02207-05
False-positive results of enzyme immunoassays for human immunodeficiency virus in patients with uncomplicated malaria
Abstract
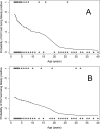
Malaria may impact upon human immunodeficiency virus (HIV) test results. We evaluated two HIV enzyme immunoassays (EIAs) by testing 1,965 Ugandans with malaria. We found poor positive predictive values (53% and 76%), particularly with younger age. Combining EIAs eliminated false positives but missed 21% of true positives. Performance of HIV EIAs in malaria may be unsatisfactory.
Figures
References
-
- Bakyaita, N., G. Dorsey, A. Yeka, K. Banek, S. G. Staedke, M. R. Kamya, A. Talisuna, F. Kironde, S. Nsobya, A. Kilian, A. Reingold, P. J. Rosenthal, and F. Wabwire-Mangen. 2005. Sulfadoxine-pyrimethamine plus chloroquine or amodiaquine for uncomplicated falciparum malaria: a randomized, multisite trial to guide national policy in Uganda. Am. J. Trop. Med. Hyg. 72:573-580. - PubMed
-
- Centers for Disease Control and Prevention. 1993. False-positive serologic tests for human T-cell lymphotropic virus type I among blood donors following influenza vaccination, 1992. Morb. Mortal. Wkly. Rep. 42:173-175. - PubMed
-
- Charmot, G. 1962. Malaria and hypergammaglobulinemia in Africa. Some recent data. Med. Trop. 22:667-671. - PubMed
-
- Chattopadhya, D., S. Kumari, R. Chatterjee, and T. Verghese. 1991. Antimalarial antibody in relation to seroreactivity for HIV infection in sera from blood donors. J. Commun. Dis. 23:195-198. - PubMed
-
- Chikwem, J. O., I. Mohammed, and T. O. Ola. 1990. Comparison of commercial kits for the detection of antibody to human immunodeficiency virus type 1 (HIV-1) in Nigeria. East Afr. Med. J. 67:209-213. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical