Galactoglucomannans increase cell population density and alter the protoxylem/metaxylem tracheary element ratio in xylogenic cultures of Zinnia
- PMID: 16891547
- PMCID: PMC1586029
- DOI: 10.1104/pp.106.085712
Galactoglucomannans increase cell population density and alter the protoxylem/metaxylem tracheary element ratio in xylogenic cultures of Zinnia
Abstract
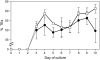
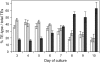
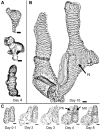
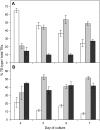
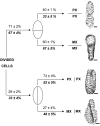
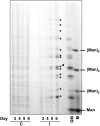
Xylogenic cultures of zinnia (Zinnia elegans) provide a unique opportunity to study signaling pathways of tracheary element (TE) differentiation. In vitro TEs differentiate into either protoxylem (PX)-like TEs characterized by annular/helical secondary wall thickening or metaxylem (MX)-like TEs with reticulate/scalariform/pitted thickening. The factors that determine these different cell fates are largely unknown. We show here that supplementing zinnia cultures with exogenous galactoglucomannan oligosaccharides (GGMOs) derived from spruce (Picea abies) xylem had two major effects: an increase in cell population density and a decrease in the ratio of PX to MX TEs. In an attempt to link these two effects, the consequence of the plane of cell division on PX-MX differentiation was assessed. Although GGMOs did not affect the plane of cell division per se, they significantly increased the proportion of longitudinally divided cells differentiating into MX. To test the biological significance of these findings, we have determined the presence of mannan-containing oligosaccharides in zinnia cultures in vitro. Immunoblot assays indicated that beta-1,4-mannosyl epitopes accumulate specifically in TE-inductive media. These epitopes were homogeneously distributed within the thickened secondary walls of TEs when the primary cell wall was weakly labeled. Using polysaccharide analysis carbohydrate gel electrophoresis, glucomannans were specifically detected in cell walls of differentiating zinnia cultures. Finally, zinnia macroarrays probed with cDNAs from cells cultured in the presence or absence of GGMOs indicated that significantly more genes were down-regulated rather than up-regulated by GGMOs. This study constitutes a major step in the elucidation of signaling mechanisms of PX- and MX-specific genetic programs in zinnia.
Figures



References
-
- Akiyama Y, Eda S, Hori M, Kato K (1983) A galactoglucomannan from extracellular polysaccharides of suspension-cultured cells of Nicotiana tabacum. Phytochemistry 22: 1177–1180
-
- Albersheim P, Darvill AG (1985) Oligosaccharins. Sci Am 253: 58–64
-
- Auxtová O, Lišková D, Kákoniová D, Kubačková M, Karácsonyi Š, Bilisics L (1995) Effect of galactoglucomannan-derived oligosaccharides on elongation growth of pea and spruce stem segments stimulated by auxin. Planta 196: 420–424
-
- Auxtová-Šamajová O, Lišková D, Kákoniová D, Kubačková M, Karácsonyi Š, Bilisics L (1996) Inhibition of auxin stimulated short-term elongation growth of pea stem segments by galactoglucomannan-derived oligosaccharides. J Plant Physiol 147: 611–613
-
- Bierhorst DW (1960) Observations of tracheary elements. Phytomorphol 10: 249–305
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

