Temozolomide chemotherapy in patients with recurrent malignant gliomas
- PMID: 16891823
- PMCID: PMC2729901
- DOI: 10.3346/jkms.2006.21.4.739
Temozolomide chemotherapy in patients with recurrent malignant gliomas
Abstract
Numerous studies have demonstrated the clinical activity of temozolomide, a second-generation alkylating agent, against malignant brain tumors, however, its activity has not been reported in an Asian population. This study analyzed the efficacy and toxicity of temozolomide in 25 adult patients with recurrent or progressive malignant gliomas after surgery and standard radiation therapy with or without chemotherapy, enrolled in our institution since July 2000. Sixteen patients had glioblastoma multiforme (GBM), six with anaplastic astrocytoma, and three with anaplastic oligodendroglioma. Of the 25 patients, 3 (12%) achieved a complete response (CR), 8 (32%) achieved a partial response (PR), 6 (24%) had stable disease (SD), and 8 (32%) had progressive disease (PD). Two patients achieved a CR, 4 patients achieved a PR, 3 patients had SD and 7 patients had PD in GBM, and 1 patient achieved a CR, 4 patients achieved a PR, 3 patients had SD, 1 patient had PD in the non-GBM patients. Median progression free survival was 8 weeks in GBM and 22 weeks in the non-GBM patients. The median overall survival of each group was 17 weeks and 28 weeks. Temozolomide demonstrated moderate activity in recurrent and progressive malignant gliomas without serious toxicity.
Figures
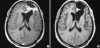
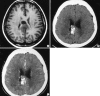

References
-
- Burger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastic astrocytoma. Cancer. 1985;56:1106–1111. - PubMed
-
- O'Reilly SM, Newlands ES, Glaser MG, Brampton M, Rice-Edwards JM, Illingworth RD. Temozolomide: a new oral cytotoxic chemotherapeutic agent with promising activity against primary brain tumours. Eur J Cancer. 1993;29:940–942. - PubMed
-
- Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846–5852. - PubMed
-
- DeAngelis LM. Chemotherapy for brain tumors. N Engl J Med. 2005;352:1036–1038. - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

