Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols
- PMID: 16893892
- PMCID: PMC1839924
- DOI: 10.1074/jbc.M603248200
Requirement of transmembrane transport for S-nitrosocysteine-dependent modification of intracellular thiols
Abstract
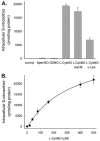
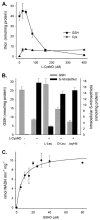
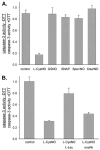
S-nitrosothiols have been implicated as intermediary transducers of nitric oxide bioactivity; however, the mechanisms by which these compounds affect cellular functions have not been fully established. In this study, we have examined the effect of S-nitrosothiol transport on intracellular thiol status and upon the activity of a target protein (caspase-3), in bovine aortic endothelial cells. We have previously demonstrated that the specific transport of amino acid-based S-nitrosothiols (S-nitroso-L-cysteine and S-nitrosohomocysteine) occurs via amino acid transport system L to generate high levels of intracellular protein S-nitrosothiols (Zhang, Y., and Hogg, N. (2004) Proc. Natl. Acad. Sci. U. S. A. 101, 7891-7896). In this study, we demonstrate that the transport of S-nitrosothiols is essential for these compounds to affect intracellular thiol levels and to modify intracellular protein activity. Importantly, the ability of these compounds to affect intracellular processes occurs independently of nitric oxide formation. These findings suggest that the major action of these compounds is not to liberate nitric oxide in the extracellular space but to be specifically transported into cells where they are able to modify cellular functions through nitric oxide-independent mechanisms.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

