Ouabain affinity determining residues lie close to the Na/K pump ion pathway
- PMID: 16894161
- PMCID: PMC1567927
- DOI: 10.1073/pnas.0602720103
Ouabain affinity determining residues lie close to the Na/K pump ion pathway
Abstract
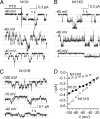
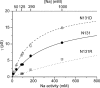
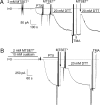
The Na/K pump establishes essential ion concentration gradients across animal cell membranes. Cardiotonic steroids, such as ouabain, are specific inhibitors of the Na/K pump. We exploited the marine toxin, palytoxin, to probe both the ion translocation pathway through the Na/K pump and the site of its interaction with ouabain. Palytoxin uncouples the pump's gates, which normally open strictly alternately, thus allowing both gates to sometimes be open, so transforming the pump into an ion channel. Palytoxin therefore permits electrophysiological analysis of even a single Na/K pump. We used outside-out patch recording of Xenopus alpha1beta3 Na/K pumps, which were made ouabain-resistant by point mutation, after expressing them in Xenopus oocytes. Endogenous, ouabain-sensitive, Xenopus alpha1beta3 Na/K pumps were silenced by continuous exposure to ouabain. We found that side-chain charge of two residues at either end of the alpha subunit's first extracellular loop, known to make a major contribution to ouabain affinity, strongly influenced conductance of single palytoxin-bound pump-channels by an electrostatic mechanism. The effects were mimicked by modification of cysteines introduced at those two positions with variously charged methanethiosulfonate reagents. The consequences of these modifications demonstrate that both residues lie in a wide vestibule near the mouth of the pump's ion pathway. Bound ouabain protects the site with the strongest influence on conductance from methanethiosulfonate modification, while leaving the site with the weaker influence unprotected. The results suggest a method for mapping the footprint of bound cardiotonic steroid on the extracellular surface of the Na/K pump.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


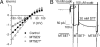

References
-
- Post R., Sen A. M., Rosenthal A. S. J. Biol. Chem. 1965;240:1437–1445. - PubMed
-
- Albers R. W. Annu. Rev. Biochem. 1967;36:727–756. - PubMed
-
- Patlak C. S. Bull. Math. Biophys. 1957;19:209–235.
-
- Vidaver G. A. J. Theor. Biol. 1966;10:301–306. - PubMed
-
- Laüger P. Biochim. Biophys. Acta. 1979;552:143–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

