Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids
- PMID: 16895428
- PMCID: PMC2531198
- DOI: 10.1021/ja061256t
Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids
Abstract
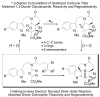
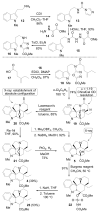
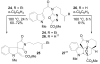
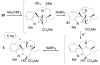
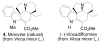
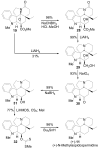
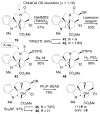
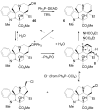
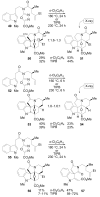
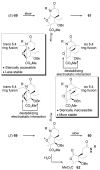
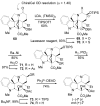
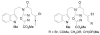
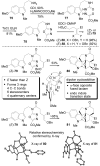
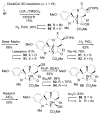
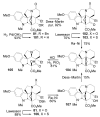
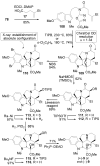
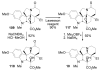
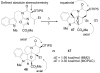
A concise 11-step total synthesis of (-)- and ent-(+)-vindoline (3) is detailed based on a unique tandem intramolecular [4 + 2]/[3 + 2] cycloaddition cascade of a 1,3,4-oxadiazole inspired by the natural product structure, in which three rings and four C-C bonds are formed central to the characteristic pentacyclic ring system setting all six stereocenters and introducing essentially all the functionality found in the natural product in a single step. As key elements of the scope and stereochemical features of the reaction were defined, a series of related natural products of increasing complexity were prepared by total synthesis including both enantiomers of minovine (4), 4-desacetoxy-6,7-dihydrovindorosine (5), 4-desacetoxyvindorosine (6), and vindorosine (7) as well as N-methylaspidospermidine (11). Subsequent extensions of the approach provided both enantiomers of 6,7-dihydrovindoline (8), 4-desacetoxyvindoline (9), and 4-desacetoxy-6,7-dihydrovindoline (10).
Figures










Similar articles
-
Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues.J Am Chem Soc. 2010 Sep 29;132(38):13533-44. doi: 10.1021/ja106284s. J Am Chem Soc. 2010. PMID: 20809620 Free PMC article.
-
Tandem Intramolecular Diels-Alder/1,3-Dipolar Cycloaddition Cascade of 1,3,4-Oxadiazoles: Initial Scope and Applications.Acc Chem Res. 2016 Feb 16;49(2):241-51. doi: 10.1021/acs.accounts.5b00510. Epub 2016 Jan 27. Acc Chem Res. 2016. PMID: 26813287 Free PMC article.
-
Asymmetric total synthesis of vindoline.J Am Chem Soc. 2010 Mar 24;132(11):3685-7. doi: 10.1021/ja910695e. J Am Chem Soc. 2010. PMID: 20187641 Free PMC article.
-
Total synthesis of (+)-vinblastine: control of the stereochemistry at C18'.Chem Rec. 2010 Apr;10(2):101-18. doi: 10.1002/tcr.201090001. Chem Rec. 2010. PMID: 20394103 Review.
-
[Development of new synthetic methods and their application to synthesis of useful compounds].Yakugaku Zasshi. 2003 Dec;123(12):1007-21. doi: 10.1248/yakushi.123.1007. Yakugaku Zasshi. 2003. PMID: 14689864 Review. Japanese.
Cited by
-
Formation and Trapping of Azafulvene Intermediates Derived from Manganese-Mediated Oxidative Malonate Coupling.Tetrahedron. 2016 Jun 30;72(26):3775-3780. doi: 10.1016/j.tet.2016.03.012. Epub 2016 Mar 4. Tetrahedron. 2016. PMID: 27551160 Free PMC article.
-
Inverse Electron Demand Diels-Alder Reactions of Heterocyclic Azadienes, 1-Aza-1,3-Butadienes, Cyclopropenone Ketals, and Related Systems. A Retrospective.J Org Chem. 2019 Aug 2;84(15):9397-9445. doi: 10.1021/acs.joc.9b00834. Epub 2019 May 23. J Org Chem. 2019. PMID: 31062977 Free PMC article. Review.
-
Total syntheses of (-)-kopsifoline D and (-)-deoxoapodine: divergent total synthesis via late-stage key strategic bond formation.J Am Chem Soc. 2014 Feb 26;136(8):3312-7. doi: 10.1021/ja500548e. Epub 2014 Feb 17. J Am Chem Soc. 2014. PMID: 24499015 Free PMC article.
-
Vinblastine 20' Amides: Synthetic Analogues That Maintain or Improve Potency and Simultaneously Overcome Pgp-Derived Efflux and Resistance.J Med Chem. 2017 Sep 14;60(17):7591-7604. doi: 10.1021/acs.jmedchem.7b00958. Epub 2017 Aug 31. J Med Chem. 2017. PMID: 28857558 Free PMC article.
-
Key analogs of a uniquely potent synthetic vinblastine that contain modifications of the C20' ethyl substituent.Bioorg Med Chem Lett. 2017 Jul 15;27(14):3055-3059. doi: 10.1016/j.bmcl.2017.05.058. Epub 2017 May 18. Bioorg Med Chem Lett. 2017. PMID: 28551101 Free PMC article.
References
-
- Neuss N, Neuss MN. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 229.
-
- Owellen RI, Hartke CA, Dickerson RM, Haines FO. Cancer Res. 1976;36:1499. - PubMed
- Pearce HL. In: The Alkaloids. Brossi A, Suffness M, editors. Vol. 37. Academic; San Diego: 1990. p. 145.
-
- Moncrief JW, Lipscomb WN. J. Am. Chem. Soc. 1965;84:4963. - PubMed
-
- Gorman M, Neuss N, Biemann K. J. Am. Chem. Soc. 1962;84:1058.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

