Ubiquitin-proteasome degradation of serum- and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP
- PMID: 16895519
- PMCID: PMC1652829
- DOI: 10.1042/BJ20060905
Ubiquitin-proteasome degradation of serum- and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP
Abstract
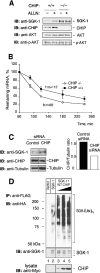
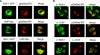
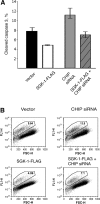
SGK-1 (serum- and glucocorticoid-regulated kinase-1) is a stress-induced serine/threonine kinase that is phosphorylated and activated downstream of PI3K (phosphoinositide 3-kinase). SGK-1 plays a critical role in insulin signalling, cation transport and cell survival. SGK-1 mRNA expression is transiently induced following cellular stress, and SGK-1 protein levels are tightly regulated by rapid proteasomal degradation. In the present study we report that SGK-1 forms a complex with the stress-associated E3 ligase CHIP [C-terminus of Hsc (heat-shock cognate protein) 70-interacting protein]; CHIP is required for both the ubiquitin modification and rapid proteasomal degradation of SGK-1. We also show that CHIP co-localizes with SGK-1 at or near the endoplasmic reticulum. CHIP-mediated regulation of SGK-1 steady-state levels alters SGK-1 kinase activity. These data suggest a model that integrates CHIP function with regulation of the PI3K/SGK-1 pathway in the stress response.
Figures



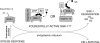
References
-
- Lang F., Cohen P. The regulation and physiological roles of serum and glucocorticoid-induced protein kinase. Science STKE 2001, RE17. 2001 - PubMed
-
- Hertweck M., Gobel C., Baumeister R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell. 2004;6:577–588. - PubMed
-
- Lamitina S. T., Strange K. Transcriptional targets of DAF-16 insulin signaling pathway protect C. elegans from extreme hypertonic stress. Am. J. Physiol. Cell Physiol. 2005;288:C467–C474. - PubMed
-
- Mikosz C. A., Brickley D. R., Sharkey M. S., Moran T. W., Conzen S. D. Glucocorticoid receptor-mediated protection from apoptosis is associated with induction of the serine/threonine survival kinase gene, sgk-1. J. Biol. Chem. 2001;276:16649–16654. - PubMed
-
- Wu W., Chaudhuri S., Brickley D. R., Pang D., Karrison T., Conzen S. D. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Res. 2004;64:1757–1764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

