West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody
- PMID: 16895988
- PMCID: PMC1567891
- DOI: 10.1073/pnas.0603488103
West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody
Abstract
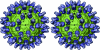
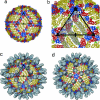
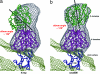
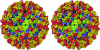
Flaviviruses, such as West Nile virus (WNV), are significant human pathogens. The humoral immune response plays an important role in the control of flavivirus infection and disease. The structure of WNV complexed with the Fab fragment of the strongly neutralizing mAb E16 was determined to 14.5-Angstrom resolution with cryo-electron microscopy. E16, an antibody with therapeutic potential, binds to domain III of the WNV envelope glycoprotein. Because of steric hindrance, Fab E16 binds to only 120 of the 180 possible binding sites on the viral surface. Fitting of the previously determined x-ray structure of the Fab-domain III complex into the cryo-electron microscopy density required a change of the elbow angle between the variable and constant domains of the Fab. The structure suggests that the E16 antibody neutralizes WNV by blocking the initial rearrangement of the E glycoprotein before fusion with a cellular membrane.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18950-5. doi: 10.1073/pnas.1011036107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956322 Free PMC article.
-
Structure of the complex of an Fab fragment of a neutralizing antibody with foot-and-mouth disease virus: positioning of a highly mobile antigenic loop.EMBO J. 1997 Apr 1;16(7):1492-500. doi: 10.1093/emboj/16.7.1492. EMBO J. 1997. PMID: 9130694 Free PMC article.
-
Structural basis of West Nile virus neutralization by a therapeutic antibody.Nature. 2005 Sep 29;437(7059):764-9. doi: 10.1038/nature03956. Nature. 2005. PMID: 16193056 Free PMC article.
-
Antibodies: From novel repertoires to defining and refining the structure of biologically important targets.Methods. 2017 Mar 1;116:12-22. doi: 10.1016/j.ymeth.2017.01.003. Epub 2017 Jan 11. Methods. 2017. PMID: 28088364 Review.
-
Immunoglobulin structure and function as revealed by electron microscopy.Int Arch Allergy Immunol. 1999 Oct;120(2):85-99. doi: 10.1159/000024226. Int Arch Allergy Immunol. 1999. PMID: 10545762 Review.
Cited by
-
Structure of immature West Nile virus.J Virol. 2007 Jun;81(11):6141-5. doi: 10.1128/JVI.00037-07. Epub 2007 Mar 21. J Virol. 2007. PMID: 17376919 Free PMC article.
-
Structure of tick-borne encephalitis virus and its neutralization by a monoclonal antibody.Nat Commun. 2018 Jan 30;9(1):436. doi: 10.1038/s41467-018-02882-0. Nat Commun. 2018. PMID: 29382836 Free PMC article.
-
Immunodominance and functional activities of antibody responses to inactivated West Nile virus and recombinant subunit vaccines in mice.J Virol. 2011 Mar;85(5):1994-2003. doi: 10.1128/JVI.01886-10. Epub 2010 Dec 8. J Virol. 2011. PMID: 21147919 Free PMC article.
-
Molecular Basis of a Protective/Neutralizing Monoclonal Antibody Targeting Envelope Proteins of both Tick-Borne Encephalitis Virus and Louping Ill Virus.J Virol. 2019 Apr 3;93(8):e02132-18. doi: 10.1128/JVI.02132-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30760569 Free PMC article.
-
Dengue viruses - an overview.Infect Ecol Epidemiol. 2013 Aug 30;3. doi: 10.3402/iee.v3i0.19839. Infect Ecol Epidemiol. 2013. PMID: 24003364 Free PMC article. Review.
References
-
- Lanciotti R. S., Roehrig J. T., Deubel V., Smith J., Parker M., Steele K., Crise B., Volpe K. E., Crabtree M. B., Scherret J. H., et al. Science. 1999;286:2333–2337. - PubMed
-
- Hayes E. B., Gubler D. J. Annu. Rev. Med. 2006;57:181–194. - PubMed
-
- Hall R. A., Scherret J. H., Mackenzie J. S. Ann. N. Y. Acad. Sci. 2001;951:153–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

